
Synopsis
This whole topic can be condensed into one statement:
Anaerobic or Anoxic Conditions do not Occur in the Home Aquarium Except in the Intestines of Fish
.
This is an in depth analysis of two related myths:
- Anaerobic conditions in a substrate allow nitrates to be reduced to nitrogen gas
- Anaerobic conditions in a substrate allow sulfates to be reduced to poisonous hydrogen sulfide gas
What is written below is a very lengthy analysis of these two anaerobic myths for freshwater aquariums. It is very long and incredibly boring but so be it. This is what is necessary to prove a negative. This article is only for real nerds like the author.
One note on terminology here. The terminology has been greatly simplified in this article. The actual definitions and limits involve all sorts of factors and indeed some very complex equations. This is all well beyond the scope of what hobbyists need to know (and what I can understand!). So this has been very much simplified.

Anaerobic Denitrification
One of the biggest myths, repeated over and over by a host of well meaning but ill-informed commentators on social media, is that conditions in some substrates (generally something called a “deep sand bed”) are anaerobic or anoxic and reduce nitrate to nitrogen gas. Now I know many will “waffle” on this topic as they do not want to offend all these commentators. I cannot do that. All these commentators are just plain wrong! Sorry, can’t sugar coat it.
.
Deep sand beds do NOT do ANY anaerobic denitrification to Nitrogen gas
.
This is true no matter how you set up the deep sand bed. Small sand particle, large sand particle, kitty litter, laterite, gravel, no plenum, plenum, very slow flow, no flow, slow flow, moderate flow, organic matter, no organic matter, three month old, three year old, etc. etc.. ALL combinations will NOT produce anaerobic denitrifying conditions. It just CANNOT happen for no less than six very solid scientific reasons.
Year long testing of four 5 inch deep sand beds showed they did NO anaerobic denitrification. We go into the six reasons and the testing in the more in depth article below.

Poisonous Gas
Another myth is that there are anaerobic or anoxic substrates which produce poisonous hydrogen sulfide gas bubbles. This is also patently false. Unequivocally:
.
Poisonous Quantities of Hydrogen Sulfide gas are NEVER produced in ANY aquarium
.
This is NOT to say that LOW oxygen conditions cannot exist in the aquarium. Low oxygen (Hypoxic) conditions can occur in an aquarium if a filter is turned off or an ornament has a deep pocket where uneaten food can accumulate well away from any water flow. If an aquarium is poorly aerated, overstocked and/or overfed this can be acerbated. Under these conditions organic bacterial toxins along with ammonia and nitrite (and some very bad smells) can be produced which can kill the fish. But it needs to be emphasized this is VERY RARE.

The Actual Science of Anaerobic Conditions
There are always some who say things like “anaerobic denitrification takes three to four years to get going in the aquarium”. Since no one is going to set up a four year experiment these commentators always get quite smug about the validity of their claim. The only practical way to disprove this claim is to look at the science behind the claim. The science says the claim is false for no less than six separate solid scientific reasons.
There is an aquarium design that’s of interest. That is an aquarium that has a very fine sand substrate of a depth of five inches or more. This is known as a “deep sand bed”. As one goes down in depth in this deep sand bed you theoretically could find the following regions IF THE SAND IS VERY DEEP, HAS SOMETHING LIKE 20% SAWDUST MIXED INTO THE SAND AND THE SAND IS VERY FINE:
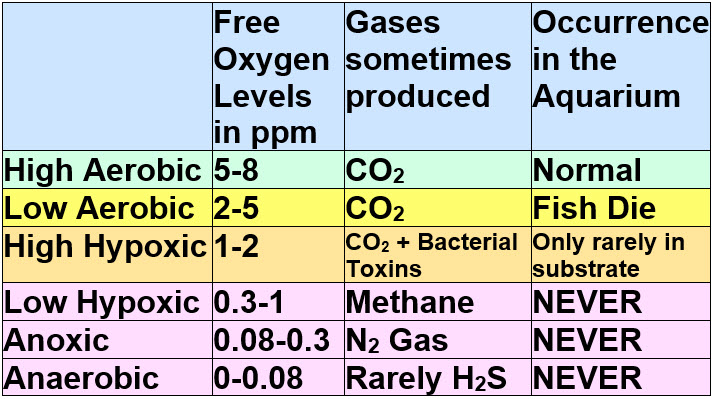
These levels are in the substrate, not in the water column. The levels in the deep sand bed substrate can be further broken down into these levels:
1, Aerobic – 2 to 8 ppm oxygen
In the aerobic region, there is lots of oxygen, with 2 to 8 ppm (mg/L) oxygen saturation. In the aerobic region, ammonia is oxidized to nitrate and organic carbon compounds are oxidized to carbon dioxide. The carbon dioxide exits the aquarium as a harmless gas. It can create bubbles. These reactions use up most of the oxygen in the substrate. These bubbles can smell bad, they are after all from decomposing sewage. Generally the lower the oxygen levels the worse the smell. Some fish get stressed at 6 ppm and die at 5 ppm oxygen while others can go down to 2 ppm before dying.

2, Hypoxic – 1 ppm to 2 ppm dissolved O2:
Marine scientist Alan Lewitus defined this zone as follows:
“Hypoxia refers to water conditions where the concentration of oxygen is so low that it is detrimental to organisms and very few organisms can survive in those conditions. Scientists refer to hypoxic waters as those waters where oxygen concentrations are below two milligrams per liter. Now, organisms that can swim away from those conditions do, they flee, and so they avoid hypoxic waters. But not always. Sometimes they’re trapped in bayments and other areas, so you see many cases where hypoxia events are associated with large-scale fish kills. In larger systems, they can flee, but you have other problems. Hypoxia can affect the habitat of fish. There’s a loss of bottom fauna, which are important food sources. Other organisms that can’t move such as shellfish and worms and so forth, are trapped and often suffocate and die.”
In low oxygen conditions (under 2 ppm oxygen saturation), where there is a protein food source, there are a host of bacteria (streptococci and clostridium being the most common) which CAN produce organic compounds which are poisonous to fish. High levels of ammonia and nitrite CAN accompany the organic poisons and contribute to fish death. But sometimes little ammonia and little nitrite are present and the fish still die.
There are literally billions of types of dark brown and black organic “humic compounds” that are commonly formed in such low oxygen conditions, forming dark colored substrate.

These hypoxic conditions produce one common denominator. They smell bad, like rotten fish and sewage.
It is not easy to get hypoxic conditions. Take the deep sand bed aquariums of Father Fish. Father Fish mixes up a mix of very rich materials like blood meal and composted manure. He then puts this mix one inch layer beneath three inches of fine-grained sand. His beds are all deep black color below one inch in depth. When Father Fish tore down some of these tanks he reported that there was no discernible odor at all. Not even an earthy smell. This is very telling.
So even Father Fish’s deep sand beds did not have hypoxic conditions after a time. Because of diffusion, Brownian movement and the movement of organisms up and down, small amounts of oxygen will still get down to the bottom of this substrate. It is just very difficult to actually stop all oxygen permeation in only three to four inches of substrate.
What people fail to realize is that water molecules do not sit motionless in liquid water. The molecules are constantly moving and colliding with one another (“Brownian movement”). This movement will take considerable oxygen down to the bottom of even a deep sand bed. If one places a plenum (think an undergravel filter with the lift tube blocked) under three inches of sand, and if one added a dye to the water column over the sand, within 24 hours that dye will have penetrated to the plenum. In the same way oxygen penetrates even deep sand beds.

It is interesting that Father Fish’s compatriot, E.J. made a Father Fish enriched soil bed with only one inch of cap. The aquarium became an hypoxic smelly cesspool and no fish could be put in it. Three inches works well, one inch doesn’t work.
When Father Fish set up his aquariums many years ago his deep beds probably initially went hypoxic. But under three inches of substrate none of the toxins or smells got into the water. As Father Fish’s tanks matured over the first few months the hypoxic conditions went away.

3, Hypoxic Fermentation – 1 ppm to 0.3 ppm dissolved O2:
In very low oxygen conditions (less than 1 mg/L but greater than 0.3 mg/L dissolved O2) there is very little oxygen. This zone is still considered by scientists to be hypoxic. Bacteria depend on the oxygen in compounds such as carbohydrates to survive. In some rare heavily planted deep bed planted aquariums with a very organic fine sand or clay substrate this may possibly occur. The organic matter contains carbohydrates which can use up the oxygen when carbon dioxide is created.
A process biologists call “fermentation” can occur in these regions (this “fermentation” only rarely produces alcohol). This “fermentation” produces organic acids such as formic acid which are then sometimes further reduced to methane gas. Tiny amounts of methane gas might be produced in such a region IF AND ONLY IF there was a lot of carbohydrates around.
Rice beds and swamps typically produce methane gas under these conditions. The gas produced is not toxic and the rice plants and swamp plants are not poisoned by the gas.

3, Anoxic – 0.3 ppm to 0.08 ppm dissolved O2
At 0.3 mg/L to 0.08 mg/L dissolved O2 there exists a condition sometimes called “anoxic” by sanitary engineers. In these area there can be reduction of nitrate to nitrogen gas IF there are some very strenuous other conditions present (electron donor, sufficient organic matter to remove oxygen, very fine media, very small amount of flow through the media chamber, lots of flow inside and around the media chamber, etc.).
This is the condition that so called “tertiary” sewage treatment plants strive for. It is simply impossible to get anoxic conditions ANYWHERE in ANY aquarium with fish in it (with the notable exception of the gut of the fish).

4, Anaerobic – 0.08 ppm to 0 ppm oxygen
In anaerobic (some biologists define this as “anoxic”) conditions there is virtually no oxygen gas (O2) (less than 80 parts per billion). This condition can occur in very organic medium which is cut off from a supply of air completely, like a sawdust and sand bed which is one foot deep. The only oxygen is in the form of sulfates. Even nitrates and nitrites are gone.
In this region, if there were high sulfates, there might possibly be tiny amounts of hydrogen sulfide produced. This might occur if one buried an egg in a very deep substrate. But note that the “rotten egg smell” of a tiny amount of hydrogen sulfide is nowhere near a poisonous amount of the gas. So the idea that poisonous amounts of hydrogen sulfide gas will be formed in a substrate in an aquarium is one huge myth. It will never, ever, happen.
Note some define anaerobic as where there is absolutely no oxygen, not even oxygen in the form of sulfates. Since this condition is completely lifeless (formation of proteins requires some oxygen to form the amino acids and life does not exist without proteins), we do NOT accept this definition.
Note also the 1 ppm, 0.3 ppm and 80 parts per billion are a very simplistic limits which are actually defined by some very complex equations by the operators of tertiary treatment plants. We won’t go there as it is all well above my lowly mathematical skills.

More on Definitions
Note the definitions of the terms “anoxic” and “anaerobic” are not very clear. Each science discipline seems to define them differently. Some biologists consider 0.3 to 0.08 ppm “anaerobic”. Some biologists consider anaerobic to be an adjective describing an organism which lives under anoxic conditions. Wastewater engineers consider this region “anoxic”. We like the definitions of Hossein Barzinmehr at the Sharif University of Technology:
“Anoxic is an adjective and it is used to describe environments without molecular oxygen (O2). Anaerobic refers to microorganism which is able to live without molecular oxygen.(Brock Biology of Microorganisms). However, in wastewater treatment field, they can be used in different ways. When we say anoxic decomposition, we are referring a condition in which microorganisms use nitrate as the terminal electron acceptor in the absence of O2. Oxidation by this route is called anoxic denitrification. In wastewater industry, a reactor that works in this way is called an anoxic reactor. Anaerobic decompositions is achieved when molecular oxygen and nitrate aren’t present and microorganisms use sulfate and other compounds as electron acceptors.
(Water and Wastewater Engineering, Davis, Chapter 22)
(Biological Wastewater Treatment 3rd, Grady, Chapter 14)”
For our purposes we will not use these definitions. Instead we use anoxic and anaerobic as synonyms meaning 0.3 mg/L to 0 mg/L dissolved O2. This is in line with what is used as definitions in the aquarium hobby, rightly or wrongly.
Note we do have several designs where anoxic filters can be made but we do not recommend their use.

What These Oxygen Levels Mean in Practice
These various levels are all based on the energy required to pull the oxygen out of various oxygenated compounds. It is easy to pull oxygen out of di-oxygen (i.e. the air), difficult to pull oxygen out of carbon dioxide to produce methane, extremely difficult to pull the oxygen out of nitrate to produce nitrogen gas and virtually impossible to pull the oxygen out of sulfates to produce hydrogen sulfide.
So denitrification is also impossible in aquarium substrates like a deep sand bed simply because the oxygen level never gets down to the 0.3 mg/l required for nitrate reduction to nitrogen gas.
There is another good reason large amounts of denitrification are impossible in a deep sand bed. Namely that the water needs to flow into the deep sand bed at the top, then flow very slowly then out the bottom of the sand bed through a plenum. No one I’ve ever seen has added a very slow flow plenum to a deep sand bed.

One YouTuber (Dr. Novak) does a slow flow through kitty litter and laterite (a “anoxic planum” or “biocenosis reactor”) in an undergravel filter. But this kitty litter and laterite substrate is so open that oxygen will permeate it quite easily all the way through. So this simply won’t work. For more on Dr. Novak’s system and why it cannot work go to this article:
8.9.1. Biocenosis Reactor
If one had some sort of very slow flow undergravel filter in the deep sand bed system (like a small nano pump turned on for seconds a day) one might possibly get some flow through and some very limited anaerobic reduction IF and ONLY IF the deep sand bed has high organic matter in it. And the sand/organic matter would have to be feet deep.
But sand simply sitting at the bottom of a tank with no nano pump system will have only the small amount of flow from Brownian movement and diffusion. If there is only tiny amounts of flow decent quantities of nitrate can’t get into the substrate to be reduced. So, while reduction of tiny amounts of nitrate might in theory take place, the amount will be inconsequential.

This small movement is difficult to understand. Let us say that one has a “Father Fish” deep sand bed in a fifty gallon aquarium. And this sand bed has 100 cubic centimeters of water, i.e. 100 milliliters or eight tablespoons of water, circulating down to the bottom of the sand bed per day. This means all fifty gallons will get to the bottom of this sand bed every 1,600 days, or every 4 years. So every 4 years the water will be cycled through and theoretically the nitrogen removed. Get the picture?
If one has a very deep, very organic substrate filled with sulfates one might possibly get a tiny amount of hydrogen sulfide gas. But it won’t be the hydrogen sulfide which kills the fish or the plants when this substrate gets disturbed. Long before the oxygen level needed to produce hydrogen sulfide is reached there will be bacterial toxins produced which will be fatal to any fish or plant in the aquarium if significant amounts of the substrate are disturbed. These bacterial toxins are just ignored in the hobby as it is just easier to blame hydrogen sulfide for deaths.

How do Tertiary Treatment Plants Do It?
Some tertiary sewage treatment plants in Europe are capable of reducing nitrates to below 3 ppm nitrates. They do this by passing the nitrate rich water into a mixing chamber which is sealed off from any air. They pass an organic compound such as ethanol into this chamber and let the bacterial oxidation of the organic compound take the free oxygen down very low.
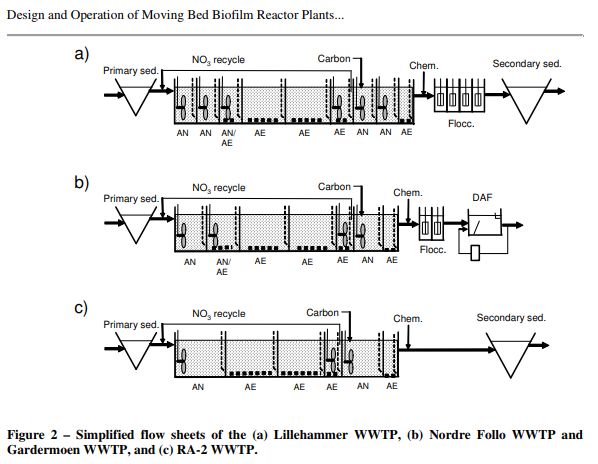
In the diagrams above the AN sections are anaerobic and sealed off from the air while the AE sections are aerobic and open to the air. “Carbon” is typically ethanol alcohol.
There is several varieties of bacteria growing on K1 type media which is rapidly circulating (bladed fan pumps are moving it) in the closed chamber. The bacteria gather many nitrate molecules in this high speed flow and use the organic compounds to “reduce” the nitrate, i.e. remove the oxygen, The resulting nitrogen gas is then passed out of the closed chamber.
Duplicating this scenario in the substrate of an aquarium is simply impossible.

Conditions Needed for Denitrification
There would have to be six conditions met for a fine substrate to do anaerobic decomposition of nitrate to nitrogen gas:
- organic matter inside the substrate
- “electron donors” inside the substrate
- impermeable to oxygen
- permeable to nitrate
- have very slow flow THROUGH the substrate
- have rapid movement of nitrate filled water AROUND the substrate

Going into each of these criteria in greater depth:
- The fine substrate would have to have large amounts of organic matter inside the substrate which would remove oxygen from the water by producing carbon dioxide gas (“anaerobic” means “without oxygen”). The maximum oxygen level for anaerobic denitrification is about 4% of the oxygen level in fully saturated oxygenated water, or 0.3 parts per million.
- The fine substrate would have to have large amounts of specific types of chemicals called “reducing agents” inside the substrate which would act as what is called an “electron donor” to reduce the nitrate to nitrogen gas through a series of nitrogen oxide intermediaries. In sewage treatment plants they typically add methanol, glycerin or ethanol to provide the electrons. In some plants they simply allow the sewage to provide the electrons but this is slower and less efficient than the other compounds.
- The fine substrate would have to be very impermeable to oxygen permeation from the surrounding water.
- The fine substrate would have to somehow pass nitrate into the substrate without passing oxygen,
- The fine substrate would need to have some mechanism by which the molecules of nitrate can be circulated VERY SLOWLY down THROUGH the substrate. In nature there is a very slow water flow THROUGH the soil to a drain of some sort. In an aquarium there is only Brownian movement and diffusion both up and down in very small quantities. Water cannot very slowly exit through the bottom of the aquarium.
- Conversely to the last requirement the nitrate molecules need to do one of two things in the lower levels of the sand. It needs to either move around VERY rapidly relative to the sand particles or spend a VERY long time there (like years). The denitrification process is a VERY low energy process which means many molecules of nitrate have to impact each denitrifying bacteria.
If ANY of these six criteria are not met the anaerobic decomposition won’t work. The first two criterion can be met by mixing in a lot of organic matter such as wood chips into a very fine, very deep (like feet!) sand substrate and letting it lie for several years. But what about the other four criteria? These four criteria cannot occur in a deep sand bed design. So denitrification is simply impossible in any deep sand bed.

“Poisonous Gas”
There are several videos on YouTube where the hobbyist disturbs the soil in their aquarium and large gas bubbles rise. One of the hobbyists claimed this “poisonous gas” (i.e. hydrogen sulfide) killed half the fish in his aquarium. The gas is harmless.
This gas is carbon dioxide created when a hobbyist uses “potting soil” from the hardware store to create a substrate for a planted aquarium. Some commercial “potting soil” is largely semi-decomposed, nitrogen fortified, ground up wood. This cellulose (chemical formula C6H10O5) is decomposed by bacteria. The aerobic decomposition produces carbon dioxide (CO2). Thus, the harmless gas. Note that this gas can smell quite bad, like raw sewage. It is after all decomposing organic matter.
Many well meaning but ill-informed commentators on social media buy into this “poisonous gas” myth. The poisonous gas they are talking about is the extremely smelly gas hydrogen sulfide, Even incredible tiny amounts of the gas, in parts per billion, can be detected by human noses. It is a characteristic “rotten egg” smell. These commentators tell you to clean and stir your substrate to prevent this “poisonous gas” (hydrogen sulfide) from forming.

In the one year test below of deep sand beds which were not stirred for twelve months, no hydrogen sulfide was detected when the beds were finally stirred. Father Fish cleaned out many aquariums with 3 inch deep “deep sand beds” with a huge amount of organic matter at their bottom which had been there for up to ten years. There was no odor at all in any of the beds. This clearly proves deep sand or any sand beds do not produce hydrogen sulfide gas.
Now there is another mechanism which CAN explain fish deaths. There are a whole host of bacteria which produce some very nasty toxins under some low oxygen conditions. If one has the power go out on a canister that has a lot of brown gunk in it, and if you start up that canister after eight to twelve hours of down time, you can kill a tank of fish. The fish die from the toxins the bacteria produce. And the tank will smell absolutely horrible. But the smell is not hydrogen sulfide. It is the smell of bacterial toxins,

Think about that, if it was easy to produce hydrogen sulfide gas, the gas would be forming in all the waterways in the world. Not only would all the fish be dead but earth would be a very smelly place to try and live.
Not even in the rice paddies and swamps, where anaerobic reduction of carbon compounds produce methane gas, is any hydrogen sulfide produced in the top foot or so of swamp mud. Now below very roughly one foot in a swamp, hydrogen sulfide may be produced in tiny quantities. But the mud is dense enough that this hydrogen sulfide will be oxidized very easily over the many years it will take for it to get into the upper layers of the soil.
Hydrogen sulfide is indeed very poisonous. If it were being produced in the mud of lakes or in aquariums it would be killing any and all plants with roots into the mud or that substrate. So aquatic plants would lead a very perilous existence if significant quantities of hydrogen sulfide were common.
There are types of sewage treatment plants which do reduce nitrates to nitrogen gas (so called “tertiary” treatment plants). These plants do not produce hydrogen sulfide gas. So even the extreme conditions required to reduce nitrates to nitrogen gas do not produce hydrogen sulfide gas.

Also note that hydrogen sulfide formation requires large amounts of sulfates to be present. In most aquariums there are only very small quantities of sulfates. Most water only has 2 to 6 parts per million sulfate.
Note that all the restrictions and prohibitions mentioned above in the section on reducing nitrates (NO3) to nitrogen gas (N2), apply equally well to the reduction of sulfate (SO4) to hydrogen sulfide (H2S).
Some say that substrate produce this hydrogen sulfide gas but that as soon as it hits the aerated water of the aquarium the hydrogen sulfide is oxidized to sulfur trioxide, which is the bad “sewage” smell people get from their aquarium. Interesting.

Oxygen (O2) is NOT a rapid oxidizer at temperatures lower than 400 degrees F, it is a slow oxidizer. It can’t oxidize something like hydrogen sulfide that quickly. It can’t happen. Sulfur trioxide doesn’t give off a sewage smell, it gives off an acrid, acidic, “burning sulfur” smell.
If one has well water and one waters their lawn, the well water will often give off the very distinctive “rotten egg” smell of hydrogen sulfide. This smell is quite distinct from the “sewage” smell frequently encountered in aquariums. Also note that if hydrogen sulfide from a well were rapidly oxidized by oxygen, it wouldn’t get as far as one’s nose!
There are a lot of organic compounds made aerobically which smell bad. The carbon dioxide gas released from pockets in organic aquarium soils smells like sewage. Sometimes very foul sewage. And people then say they have poisonous hydrogen sulfide gas. They don’t have hydrogen sulfide. They simply have rotting organic matter. BIG difference.

Scientific Testing of Anaerobic Substrates
An excellent well run study proved beyond any doubt that “deep sand beds” do NOT do any denitrification to nitrogen gas.
This study was on some 27 salt water tanks was run twice (54 runs) (“An Experimental Comparison of Sandbed and Plenum-Based Systems. Part 1: Controlled lab dosing experiments”, Robert Toonen 2005). They had three variables, depth of the sand, particle size of the sand and the presence of a plenum under the sand. They found no difference in nitrate levels in all 27 tanks after 114 days.
“Time-series of ammonia, nitrite and nitrate concentrations in aquaria showed little difference among treatments. After the initial 21d, there were no significant differences among ammonia, nitrite, nitrate, pH or salinity measured for any treatments through the end of the experiment. Analyses of variance also revealed no significant differences among the final 114d concentrations of ammonia, nitrite, nitrate, oxygen, organics or salinity, nor were there any significant interactions among experimental treatments for any of these water parameters.”

In the second part of this well run experiment 27 aquariums were set up with live fish. Over a span of 116 days there was no difference in nitrate with the different treatments:
“Time-series of ammonia, nitrite and nitrate concentrations in aquaria showed little difference among treatments. As with the dosing experiments presented in Part 1, the time-series of pH, salinity, ammonia, nitrite and nitrate concentrations in aquaria showed no significant differences among treatments. Analyses of variance for each water parameter revealed no significant differences among the final salinity, ammonia, nitrite, nitrate, oxygen, or organic concentrations, nor were there any significant interactions among experimental treatments for any of these water parameters”.
This study did note that, in the 27 aquariums with animals put in them, the shallow sand beds had double the deaths of the deeper beds. But the author quite correctly noted there was no clear “cause and effect” and did not consider it “significant”. In a study with many inputs and many outputs such as this, it is quite normal to get a correlation between two variables just by chance.

For instance if one has three inputs and seven outputs and one is looking at a 95% “significance level”, there is about a 85% chance of getting at least one “significant” correlation which is actually not a “significant” correlation.
This was 54 aquariums run in a very good experimental design with some very sophisticated technology, much beyond what the author did with buckets in the study below. This Toonen study probably applies equally well to freshwater as the mechanisms of nitrification and denitrification do not change from saltwater to freshwater.
This superb study pretty well debunks the anaerobic sand bed myth in and of itself. But some have criticized this study as only encompassing 114 days. The claim is that it takes much longer for a denitrification to nitrogen gas ecology to be set up. The following is a year long study we did in buckets which puts that theory to rest.

Test of Deep Sand Bed Denitrification
A YouTuber named Jay did some experiments using small, two liter bottle freshwater “deep sand beds” and a control with large pea gravel. All his set-ups showed zero nitrate after six months, including the pea gravel. Jay was very honest and sincere and actually did a very good, well thought out experiment.
Unfortunately Jay had the common malady of “experimenter bias” and incorrectly concluded the test “proved” anoxic decomposition of nitrates to nitrogen gas occurred with ease. His test actually proved just the opposite.
So we decided to somewhat duplicate his test only to run it to completion with digestion as the final step, add a better “control” and do it on a larger scale.

Abstract
Ten buckets with various types and set ups of deep sand beds were set up. Two were well aerated controls with no substrate where no denitrification could possibly take place. All ten buckets were subjected to the same 12 months of running with sizable food additions and no water changes and 3 months of “digestion” running designed to convert everything to nitrate. At the end of the test all ten tests gave similar very high levels of nitrate, indicating no denitrification had taken place in any of the buckets.
None of the buckets, when the sand was stirred at the end of the test, gave off any of the characteristic hydrogen sulfide smell. I.e. no hydrogen sulfide was produced.
The conclusions of this test were:
- No anaerobic or anoxic decomposition of nitrate to nitrogen gas took place.
- Low protein food gave a lot of assimilatory denitrification.
- High protein food gave significantly less assimilatory denitrification.
- No hydrogen sulfide was produced
One should be cautious about trying to read too much into this test. The nitrate tests were not as accurate as one would like. There are NO statistically significant relationships beyond what are listed above.

Test Procedure
Ten 5 gallon buckets were set up. Two foods were selected, a low protein, high carbohydrate food (Wardley goldfish flake food, 29% protein, 53% carbohydrates), and a high protein, low carbohydrate food (Purina Aquamax 300, 50% protein, 14% carbohydrate).
Five substrates were set up:
- One inch of fine play sand
- fives inches of fine play sand
- five inches of fine play sand with 10% sawdust mixed in
- five inches of pea gravel
- no substrate.
Each of these substrates were done to two buckets.
The “controls” were two bare bottom buckets with powerhead operated Poret sponge filters and air stones (no anaerobic conditions are possible in these two buckets).
Each bucket had three gallons of water added to it. The water was RO water of pH 6.8, GH of 1 and KH of 1. Each bucket had about one half cup of brown gunk squeezed from an established sponge filter added at the start to make sure all ten buckets had the same bio fauna and bacteria.

The five buckets fed the 29% protein food had 4 grams of flakes added per week. The buckets fed the 50% protein food had 2 grams of pellets fed per week. This difference in quantity of food reflects the differences in nitrogen content. In this manner the amount of nitrate in the buckets should be equal after digestion if no denitrification took place. The buckets were placed in a room at 72 degrees. No water changes were done for the entire test. pH was not adjusted for the first 12 months. RO water was used as the make up water. The testing gave the following matrices:
Before testing:
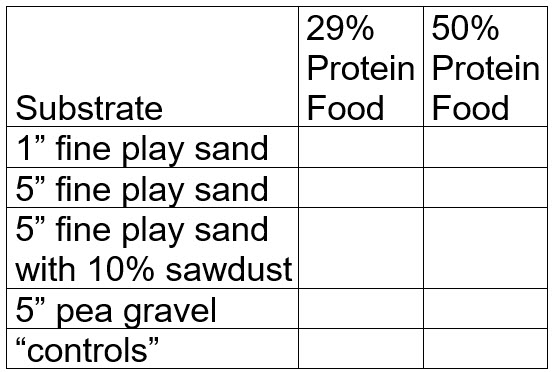
.

After three months the levels of nitrate in the buckets were
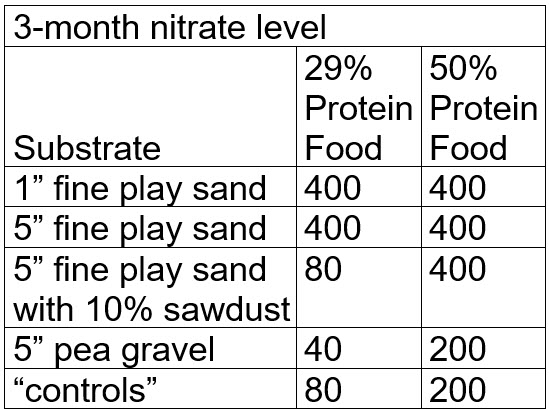
.

After six months the nitrate levels were:
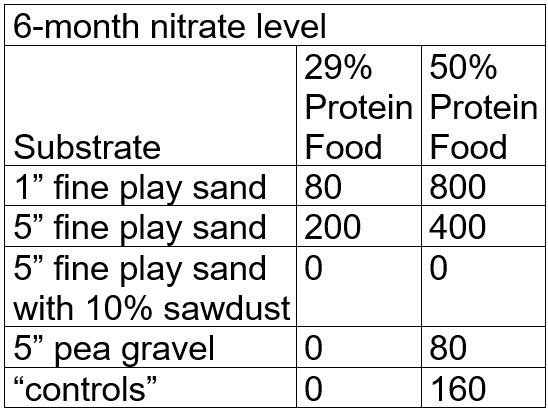
.

After nine months the test results were:
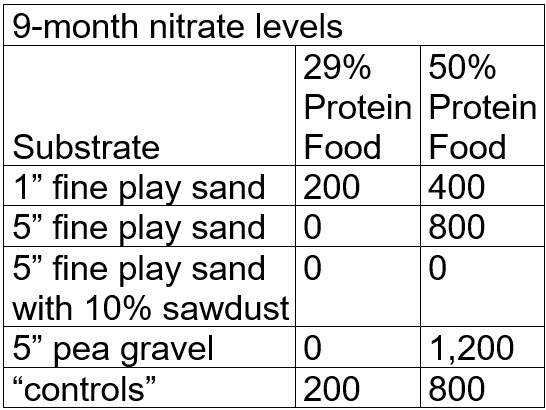
.

After 12 months the nitrate levels were:
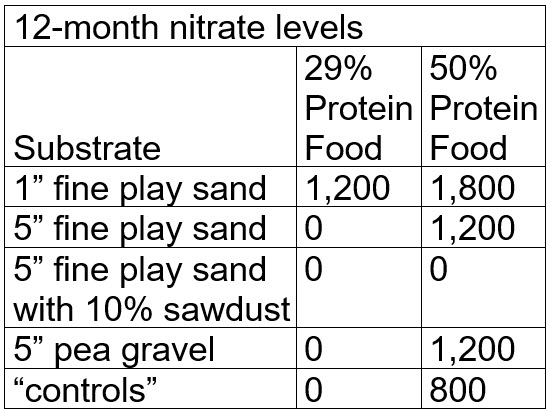
Note that at this point many testers would stop the test and loudly proclaim that the test “proved” denitrification of nitrate to nitrogen gas was occurring in the most of the buckets. But what actually happened was that the nitrate was bond up in the black and brown gunk (“mulm”) in each bucket. This is called “assimilatory denitrification via mulm”. To prove this it is necessary to “digest” all the mulm.
Using assimilatory denitrification via mulm to remove nitrate from an aquarium is possible but tricky. The whole topic of assimilatory denitrification via mulm has an entire article devoted to it.
8.9.3. Assimilatory Denitrification

Note that the nitrate levels seen in the “controls” were surprising and unexpected. Both the “controls” built up a heavy layer of brown gunk on the bottom of the buckets, even though they were well aerated. Conventional wisdom is that at least the high protein bucket should have had this brown gunk oxidized and high nitrates throughout the test. But Mother Nature will sometimes throw a curve. We suspect this “curve” was due to acidification preventing decomposition.
The buckets than had a “digestion” period where the buckets were well aerated and the pH was adjusted up to 7.4 with powdered sodium bicarbonate. After three months of digestion and pH adjustment the results were:
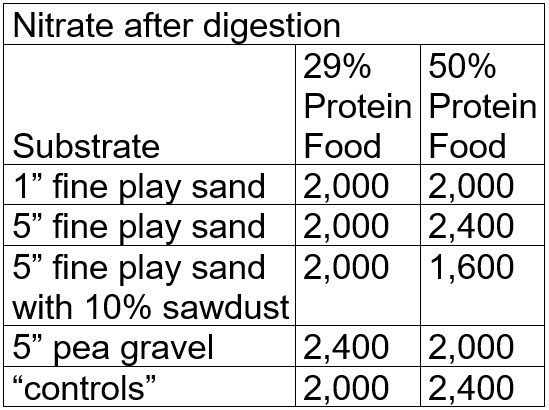
There is no significant difference in the levels of nitrate seen after three months of digestion. The levels of nitrate in the buckets which could not possibly do any denitrification (the “controls”) were statistically identical to the levels seen in the other buckets.
Test Details
Three gallons of water was added to each bucket. The water levels in each bucket were marked and topped off with distilled water once a week. The buckets were covered with black plastic so no algae could form. The buckets were fed once a week for six months, just as Jay did. Since this was an easy “hands off” test with little maintenance required, another six months of feeding was done.
At the end of twelve months the feeding was stopped. The substrate in all ten buckets was then well stirred to produce a brown soup.

When each bucket was initially stirred after one year of no movement at all in the substrate, the buckets were smelled. While the buckets without aeration smelled like very bad sewage they did not give even a hint of the very characteristic hydrogen sulfide smell. Since the human nose can test hydrogen sulfide in the parts per billion range it is safe to say none of the deep sand beds produced any hydrogen sulfide gas.
Because of the smell the buckets were moved to a garage. It was summer in Florida so the temperature in the garage was 85 to 95 degrees for the digestion portion of the test. The pH was brought up to 7.4 to 7.9 with addition of powdered sodium bicarbonate, CONSIDERABLE sodium bicarbonate.
The eight un-aerated buckets were then aerated with airstones overnight. The buckets still smelled very bad the next morning. Two bait fish were then added to each of the ten buckets. In the eight buckets which had not been aerated for one year all the bait fish died within one hour. These bait fish probably died from the organic poisons put out by bacteria which live in rotting food under low oxygen conditions. Bait fish put in the two controls did not die.

After the bait fish test, the buckets were modified to “digest” all the nitrogen compounds to nitrate. Each bucket had an Imagitarium bubbling ring airstone (4″ diameter) buried below the substrate attached via tubing to a large common air pump. Each bucket also had a power head operated Poret foam sponge filter added. The powerhead in each bucket was aimed in such a way as to produce a circular current in the bucket.
The substrate was stirred frequently in each bucket, the walls of the bucket were scraped, the Poret foam was squeezed frequently and the pH level of 7.4 to 7.9 was maintained.
This “digestion” testing was then continued for three months. These three months were designed to convert ALL the various forms of nitrogen (protein, amines, DOCs, ammonia, nitrite, and nitrate) in the buckets to nitrate. In this way one can determine if any nitrogen had gassed out as nitrogen gas.

At the end of three more months very little “brown gunk” remained in any of the buckets so the test was concluded.
The level of nitrate was measured with the API master test kit. Ten milliliters of solution were pipetted into a large graduated cylinder and distilled water was added to bring the amount of solution to 800 milliliters. The API nitrate test was then run. The results above showed no significant difference in the nitrate levels of all ten buckets, indicating no denitrification had taken place.
Because the author is conducting some tests on test kits for the home aquarium, he had some other nitrate test kits. So, he tested the ten buckets with six more test kits (Onthink, Salifert, Seachem, API 5 in 1 Test Strips, Stript Health Test Strips and Hach Test Strips). All six tests gave similar values using a dilution method. Note the exact number varied considerably between all the test kits. But the end value across all ten buckets were very close within any given test.
This is a very easy test that anyone can do. This was very deliberate in order to allow those who are interested to easily duplicate the test. Any hobbyist so inclined is encouraged to do just that.

The Math
A sanity check is in order on the nitrate levels.
- 2 grams of food per week
- 52 weeks
- 50% protein
- 13% of protein is elemental nitrogen
- elemental nitrogen needs to be multiplied by 3.36 to get grams nitrate in each bucket
- (2 x 52 x 0.50 x 0.13 x 3.36) = 22.7 grams
- 3 gallons of water
- 22.7/3 = 7.57 grams per gallon nitrate
- one part per million is 0.37 grams in a 100 gallon tank
- (7.57 x 100) / 0.37 = 2,046 ppm nitrate
This is very close to the total seen at the end of the testing. So again there was no removal of nitrates by reduction to nitrogen gas.

Similar Tests by Others
There was one test which simply tested a single unplanted aquarium and got no denitrification with a deep sand bed.
There is a whole series of YouTube videos put out in September and October of 2021 and February 2022 by Dan Hiteshew (Everyday Fishkeeping). He has a small aquarium where he had about a four inch deep sand and gravel bed. He added well water with 20 ppm nitrate every water change and fed a bunch of fish. He then watched the nitrate levels in the “deep sand bed” aquarium. Here is Dan with the small aquarium.

Dan did not find any reductions in nitrate in this tank over the span of six months. He still had to do regular water changes to keep the nitrates down.

“Expert” opinions on Anaerobic Reduction of Nitrates to Nitrogen
Many “experts” contend that conditions in a home aquarium can reduce nitrate to nitrogen gas. Per the Aquaworld Aquarium eBook “Mastering Freshwater Aquarium Ecosystems”. by Tony Griffitts (note this website now has ransomware imbedded in it so I don’t recommend looking at it):
“In the lower depths of the substrate where very little water is exchanged, an anaerobic (no oxygen) environment develops, that is the primary habitat of anaerobic bacteria. Anaerobic bacteria use the oxygen in nitrate to respire. The anaerobic bacteria that breaks down the nitrate will eventually release nitrogen gas (non-toxic) that will bubble out of the aquarium.
Fine grain sand will develop an anaerobic layer at a shallow depth in as little as ¼ inch (6 mm) in depth. The larger the substrate size, the greater the depth needs to be before the aquarium ecosystem can benefit from denitrification. Larger substrates marketed for aquarium use may require several inches before an anaerobic layer will develop.”
This can’t be sugar coated. This is simply patently false. It is fake science. Has never happened and will never happen. The test above shows it is false. Here is an in depth analysis which shows why it is false:

Misinterpreted Anecdotal “Evidence” of Anaerobic Denitrification by Deep Sand Beds
There are some YouTube videos claiming an aquarium that has not had water changes for a long time (seven years in one case) and has low nitrates because of its deep sand bed. The mechanism here is that the aquariums had algae, plants and juvenile fish growing in them as fish breeders grow out tanks. The plants and algae absorbed the nitrates and the fish ate the algae and plants. The fish and plants incorporated the nitrogen into their bodies and removed it when they were sold off or died and were removed from the tank.
The other mechanism which removes nitrates is simply cleaning the filter. The brown gunk in the filter has nitrogen tied up in it, sometimes considerable amounts of nitrogen. So every time the filter is cleaned nitrates are removed from the aquarium, especially if a low protein food is being fed the fish.

The ONLY other “research” found which “proved” denitrification was anecdotal single aquarium “experiments” where the reduction in nitrate can be explained by several different assimilatory denitrification pathways. “Assimilatory denitrification” is the well established removal of nitrogen compounds from water by incorporation of the nitrogen into cell proteins in fish growth, bacterial mass growth, algae growth and plant growth.
The whole topic of assimilatory denitrification has an entire article devoted to it.
8.9.3. Assimilatory Denitrification
There are also a whole host of well meaning but ill informed YouTube videos which extol the nitrate reducing virtues of deep sand beds. Almost ALL of these videos are heavily planted tanks. The plants absorb the nitrates as they grow. This mechanism is quite simple and quite well documented.
Also the bacteria, water molds and fungi growing in the sand bed absorb nitrates as they grow. The effect of this can be considerable. The biomass in a deep sand bed can be considerable and can contain equally considerable amounts of nitrogen. This process is called “assimilatory denitrification” and is VERY common.

The uptake by fungi, water molds and bacteria of nitrate is called “assimilatory denitrification”. It is common and well documented. Per the paper “Denitrification in Recirculating Systems: Theory and Applications” van Rijn et. al 2005:
“Biological nitrate removal is conducted by a wide variety of organisms by either assimilatory or dissimilatory pathways. Organisms capable of assimilatory nitrate reduction use nitrate, rather than ammonia, as a biosynthetic nitrogen source. Organisms capable of assimilatory nitrate reduction include plants, algae, bacteria and fungi. Assimilatory nitrate reduction takes place under aerobic as well as anaerobic conditions. No net removal of inorganic nitrogen is accomplished by this process, since inorganic nitrogen is converted to organic nitrogen”
The nitrate is NOT being reduced to nitrogen gas in the sand bed. The nitrogen is simply being incorporated into biomass.

The well run experiments above on salt water deep sand beds (“An Experimental Comparison of Sandbed and Plenum-Based Systems. Part 2: Live Animal experiments”, Robert Toonen 2005) showed this assimilatory denitrification quite well:
“Overall, the concentrations of nitrate recorded throughout the live animal experiment are significantly lower than those reported for the dosing experiments (similar to the results for ammonia and nitrite)”.
The 27 aquariums dosed without animals in them had 50 ppm nitrate at the end of the tests and the 27 aquariums with animals in them had 5 ppm nitrate. This is due to assimilatory denitrification by the live fish. Note this was an incredibly well run scientific test where no one can take issue with how it was done nor how solid the conclusions were.

Also note that the bacteria which oxidize ammonia to nitrate require high oxygen conditions. In a substrate where the oxygen conditions are less than 50% saturation ammonia is not oxidized to nitrate. So there can be considerable nitrogen tied up as ammonium in deep sand beds. There are also some very stable brown and black organic acids called humic acids which can permanently tie up considerable nitrogen in their structures.
In areas of LOW oxygen (under 20% saturation) there are a whole series of bacteria which produce organic toxins which can rapidly kill both humans and fish. The toxins CAN include ammonia and nitrite but sometimes neither ammonia or nitrite are present in sufficient quantities to kill.

If a canister filter loaded with “brown gunk” stops working (think a child unplugging it or a power outage) for more than 4 to 8 hours the brown gunk will “sour” with these low oxygen bacteria. Unsuspecting hobbyists who then turn the canister back on will kill their fish with very “sewage” smelling poisons.
The bait fish experiment proved these toxins do indeed kill fish. But these toxins are not produced by ZERO oxygen conditions. They are produced by LOW oxygen conditions. There is a big difference. Nitrate reduction to nitrogen gas and hydrogen sulfide production both require VERY VERY low oxygen concentrations.

Jay’s Experiment
Above we mentioned Jay’s experiment on denitrification. Here is a deeper analysis of his experiment. Jay’s YouTube video (Jay’s Aquarium, https://www.youtube.com/watch?v=_gYIZ_O_D_o) compares three depths of sand beds (1.4 inches, 2.9 inches and 5 inches) with a five inch deep bed of half inch gravel.
Over the span of six months with weekly feeding, no fish, no water changes, no plants and no light, the gravel (and all three sand beds) had consistently low nitrates, going to zero in three months and staying there, despite continued feeding. The deep gravel was significantly faster to zero nitrate than the deep sand bed. This is because the bacteria, water molds and fungi had perfect growth conditions in the gravel. The nitrates were NOT reduced to nitrogen gas, they were absorbed in biomass growth, black humic acid formation and ammonium production in acid conditions.
The uptake by fungi, water molds and bacteria of nitrate is called “assimilatory denitrification”. It is common and well documented. See the reference above.

Note that the deep sand bed cylinder also went to zero. It just had a higher nitrate peak and took four and a half months to come to zero. So there was considerable fungi and bacterial growth in the top layers of the “deep sand”. This black color is from nitrogen rich humic and fulvic acids. But both the shallow sand beds also came to close to zero after four to five months of operation. The walls of the cylinders in the shallow sand were dark brown in color, indicating lots of humic acid formation.
Nitrogen rich brown and black humic acids form in acid water. Oxidized food is inherently acid. So the nitrate levels in the cylinders was somewhat dependent on acidity. This is why the tanks only started dropping rapidly in nitrates after a period of time had passed, typically two to three months.

Jay did not have any fish in his test cylinders. He added cheap goldfish food that was probably 50% carbohydrates. Normally the carbohydrates in fish food are burned up by the fish’s metabolism. In this case the carbohydrates were being used as a source of energy by fungi, water molds and bacteria for converting nitrate into proteins and organic acids. This was forming a nitrogen rich biomass full of nitrogen rich black humic acids. The nitrogen rich, acid rich biomass was obvious as a thick black mulm in the gravel and brown or black coating on the walls of the cylinders.
The cylinders had the top surface of the water completely exposed to the air. The oxygen exchange at this surface was far more than was needed to oxidize the carbohydrates in the food. Anaerobic conditions can ONLY exist when there is more carbohydrates than oxygen in the water and ONLY when the oxygen supply is incapable of being replenished.

The oxygen permeates the water column via the same mechanism that the dissolved organic compounds will permeate the water column, by currents and Brownian movement. The water will evaporate at the surface. The evaporation will cause the water to cool. The cooler water is denser water and will sink. And currents are set up.
To test water movement in Jay’s gravel cylinder we set up an identical cylinder with five inches of pea gravel, just like Jay. We filled the cylinder with water. We then added five drops of red food coloring. In five hours the water at the bottom of the cylinder was the same pink color as all the other water in the cylinder. This is an easy test anyone can do.
Jay claimed that “obviously” the entire water column had gone anoxic in the shallow sand cylinders as the water “went foul and smelled nasty“. Thus “even a 1.4 inch sand bed had removed nitrates by anoxic reduction to nitrogen gas“. Newsflash, rotting food smells bad when the oxygen level drops to below roughly 50% saturation.

The tubes with the shallow sand had much more volume of water in them than the tubes with the five inches of gravel and the five inches of sand. Each tube had the same surface area exposed to the atmosphere. So, in Jay’s experiment, the oxygen level in the shallow sand beds dropped below 50% and the oxygen levels in the deep gravel and the deep sand did not drop below 50% due to the ratio of the surface area to the volume. So the shallow sand beds smelled and the deep sand beds didn’t smell.
And the deep gravel and deep sand did not go “foul and smelled nasty”. Yet somehow the deep gravel and the deep sand “went anoxic” per Jay and reduced the nitrate to nitrogen gas better than the shallow sand cylinders.
Note that Jay had a completely opposite take on the situation. He said that obviously the half inch gravel gave very good anoxic conditions as did all the cylinders including the shallow sand. He then said that it was obvious that it was much easier to get anoxic conditions than one would surmise. In actuality his experiment proved exactly the opposite.

Jay set up his experiment with a “control”. His gravel was his “control” and the fact that his “control” reduced the level of nitrates faster than his “deep bed” cylinders, should have led Jay to realize he was proving deep beds didn’t work. Five inches of half inch gravel CANNOT POSSIBLY go anoxic.
But Jay did a very good job of setting up his experiment and is to be commended for that. He just understandably misinterpreted some points in his conclusions (very few hobbyists know about “assimilatory denitrification”). This is a VERY common phenomenon called “experimenter bias”. When we expect something to be proven we tend to find a proof where there is no such proof. I’ve found myself doing it on more than one occasion.

Well Water Nitrates
The simple proof that a five inch substrate can’t reduce nitrates to nitrogen gas is provided by the fact that there are many wells down to 200 feet in depth in farm country that have nitrate concentration in the 50 to 300 ppm range. At depths deep in the water table there is no question there is no oxygen. Yet the nitrates aren’t being reduced to nitrogen gas.
Black Substrate
There is also a myth that if you can see black substrate through the aquarium glass you have anaerobic conditions. A point from the gardening world is pertinent. When a gardener adds compost and organic material to his soil he is trying to get “humus”, a black aerobically formed material made up largely of humic acids. This black soil material is “black gold” to an experienced gardener.
Humic acids have a very high “cation exchange capacity” (CEC). Which means they can absorb a large amount of ammonium and tie it up.

This humus is the same material an aquarium owner sees in his white sand substrate. It is harmless and does not indicate anaerobic conditions. It does indicate acid conditions. Beneficial bacteria filled detritus starts out light brown and slowly darkens to a dark brown to black color as humic acids accumulate. This is beneficial bacteria filled detritus and it is GOOD in the aquarium, not bad.
One of the more experimental people in the DSB (“deep sand bed”) community in 2009 was DeeDeeK, who buried her dead dwarf Gourami in the Deep Sand bed to see what would happen. The sand in the location and around it went jet black for a period of time, and then slowly faded to normal color. Many said this black was an anoxic pocket that reduced the ammonia from the decomposing fish to nitrogen gas. They were simply wrong.
It was an aerobic pocket where the fish decomposition produced black acidic organic compounds like humic and fulvic acids under conditions of LOW oxygen and somewhat low pH. As the oxygen from the water continued to permeate the pocket over time, the various acidic black compounds were oxidized to carbon dioxide and all the nitrogenous compounds from the fish decomposition were oxidized to nitrates. And the black color disappeared.

Research Papers
There are some research papers out there which conclude nitrogen is easily removed as nitrogen gas from all freshwater bodies. There are also a host of papers, some quite recent, which say denitrification to nitrogen gas occurs in as little a 1 millimeter of muck. One paper even said the process of oxidation to nitrate and the process of denitrification to nitrogen gas occur side by side in a biofilm.
There is a political situation which has been going on for some seventy years. Huge agricultural companies are under attack for the amount of nitrates their fertilizers put into the environment. These companies give large sums of money to politicians’ campaign funds. These politicians have control for the funding for research at many universities. And these universities are coming out with papers which say nitrates are easily reduced to nitrogen gas in the environment and are thus not a problem. And everyone then says nitrates cannot cause toxic cyanobacteria blooms or other ecological problems. LOL Do you see the process here?
In 2012 I went to one lecture by a University of Florida scientist on the ecology of Lake Okeechobee, which is quite close to my house. I ask the scientist what the nitrate levels were in the lake. She said they didn’t measure it “Because it fluctuated too much to be meaningful“. Get the picture? I’m trying to picture the response I would get if I replied to somebody talking about nitrate levels in the aquarium with “don’t bother to measure it as it fluctuates too much“. LOL

Note that a fine sand bed can go hypoxic under certain conditions. The conditions include some combination of:
- fine filter sand or play sand
- no pond mud inoculate
- little aeration
- overstocking
- low protein food
- heavy feeding
- low water movement
Very rarely some combination of these factors can create hypoxic conditions in the substrate which release irritating chemicals into the water column and affect the fish.

An Example of Hypoxia:
I had a question and answer in the comments section on this website which I think illustrates hypoxia in a substrate perfectly. So I’ll just repeat the exchange here:
Jim Mc
I would like to run something by you concerning hypoxia in the aquarium. For years I had issues in my 265 gallon African cichlid tank with the fish flashing constantly to the point they would injure themselves. They also would have what I can only describe as a spasm of some sort where their body would twitch, fins quiver and dart around the tank. Some fish exhibited this behavior more than others, usually the smaller or less dominant fish. I tried every medication or remedy I could find over the years thinking I was dealing with some sort of external parasite. Obviously, I would do a treatment for the recommended period of time then add carbon and do a large water change before I tried something else. Nothing worked. I finally came across a local veterinarian who had experience with all sorts of exotic animals, including fish. I brought one of my yellow Labidochromis to him. He clipped a sample of the gills and also took a skin scrape and couldn’t find anything noteworthy.
I decided to have a full necropsy done on the fish through Cornell University. Results came back negative for anything parasitic, bacterial or fungal. I thought maybe I didn’t bring him a specimen that was fully symptomatic so I tore my tank apart to catch three more fish that I knew had persistently exhibited the flashing and spastic behavior. 3 more necropsies done and no significant findings from any of them. At this point, I know it has to be something in the tank that is causing it. I have two other tanks with African cichlids with the same water quality and no issues. The only objects in the tank are a couple hundred pounds of lace rock, some artificial plants, pool filter sand and eggcrate that I had placed underneath the rocks to help distribute their weight and provide support.
Fast forward a couple months to July 2021 and my tank starts leaking from a corner. I do a full tear down and eventually rehome the majority of yellow labs and move the rest of my haps and peacocks into a new 125. When I had the 265 almost fully drained there was a horrible stench similar to what you described as sewage in your article. There was sand caked solid in the holes of eggcrate that was dark brown in color, much darker than the normal, whitish appearance of the pool filter sand. It has been 7 months since the fish have been in the 125 and they have yet to display the flashing or spastic behavior. From the information I have given you, would you feel comfortable confirming I had a hypoxic substrate and that’s what could be causing the behavior I was seeing?
I would also like to note I never experienced many fish deaths over this time, maybe one or so a year. My thought was because I frequently changed large amounts of water the toxins weren’t prevalent throughout the tank at high levels and I also never moved the rocks, sand or eggcrate during this time. As noted earlier, it seemed that juvenile and less dominant fish were affected more than adult, dominant fish so perhaps their immune system was more stressed making them more susceptible to the bacterial toxins. Thanks again.
.
Dave
In reply to Jim … Sure sounds like it. That sewage stench is a dead give away. I’d be interested in some points. Do you think the stocking was heavy? Was the aeration good? Could you have been overfeeding? Any indication the food was low in protein? I’m trying to understand the conditions that will give a hypoxic substrate.
.
Jim Mc
Dave, definitely overstocked for sure. About 60 yellow labs ranging from fry to full grown adults, ten 4- 6 inch haps and peacocks, 4 large clown loaches 6- 9 inches, six 5 inch synodontis multipunctatus and 2 bristlenose plecos. I would say I have a heavy hand when feeding as well. The food was a combination of NLS, Northfin and Extreme so I believe all less than or about 40% protein. That was before I found your site. I have since switched to the Lakeway Tilapia feed that you recommend. I had 3 Fluval FX5’s and a large sponge filter as well as 2 wavemakers, one on each side. I added the wavemakers and sponge filter because I didn’t believe I and enough aeration or movement. I know the lace rock trapped a lot of food and feces as well due to all the crevices. Definitely not enough movement on the substrate as feces would collect in certain spots.
Normally I avoid anecdotal evidence but this was so clear cut I had to include it in the discussion. Definitely an hypoxic substrate.

Proving a Negative
Note that this whole very long aquariumscience.org article has one big problem. Namely that it is impossible to prove a negative. No matter how many papers or tests I can come up with there will always be those who say “what about this or that set of circumstances, no papers cover that“. Thus the verbosity.
Further Research
For more on the anaerobic myth click on the following:
14.2.4. The Anaerobic Substrate Myth
7.5. Denitrifying Media
8.9. Anaerobic Reactors
.
Return to Equipment Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!

Dave says
In reply to Jeremy r ….. The kitty litter “anoxic plenum” by Dr. Keven Novak does not work, as confirmed by yearlong testing with controls. See this article for the complete test and analysis. The analysis shows why the reactor does not work and cannot work. The analysis also explains why so many people say they have had “success” with the reactor. It is something called “assimilatory denitrification”, generally meaning “absorption of nitrates by plants”. https://aquariumscience.org/index.php/8-9-1-biocenosis-reactor/
Jeremy r says
Hi Dave,
I’ve recently come across this Dr (of some speciality) on YouTube talking about plenums with very slow flow, creating anoxic conditions and completing the nitrogen cycle.
Based on what you’ve documented above, his claims would be unlikely.
https://youtube.com/@anoxicfiltrationplenums?si=lisQnG_i2XOxZBa3
He does refer to sewerage treatment.
Most of his videos use fine gravel, a few inches and an empty area under the plenum with a very slow flow, 10x gravel volume per day of water flow.
He also talks about achieving an orp above 300, presumably this is due to additional substrate additives he uses which is aiding in that process.
Your thoughts?
Cheers
Dave says
Biohome and Seachem “De*Nitrate” (Matrix) were tested over a period of one year and did not do any nitrate reduction. They were tested against two plastic media “controls” which couldn’t possibly do any nitrate reduction and there was NO difference in nitrate levels after a year, even though very low flow filters were included in the tests. Biocenosis baskets were also tested and didn’t work. This article includes an extensive analysis as to why reduction of nitrate to nitrogen gas is impossible in the aquarium.
http://aquariumscience.org/index.php/7-5-denitrifying-media/
Ola says
Your thoughts on cannister media’s as “BioHome”:
“.. and 4-6 months for the inner non oxygen surfaces to to colonise by anaerobic Nitrate converting Bactria
( flow rate is a major factor in the reduction of Nitrates)”
Sounds great..?
Dave says
In reply to Kenneth ….. There are two good ways to remove nitrates: 1, water changes (I like drip continuous changes for lots of tanks) or 2, large areas of plants. I highly recommend a drip system for you. Don’t even think of going to a denitrification via a tertiary sewage treatment plant method. Treatment plants need all sorts of controls to do it. https://aquariumscience.org/index.php/18-2-drip-water-changes/
Kenneth Manning says
I’ve never used a deep sand filter becaise the thought of risking massive fish loss just to avoid a few water changes is crazy to me. My question pertains more to the nitrate to nitrogen gas part of the article. I’ve been dabbling in fish breeding as a hobby for a little while, with a fairly good degree of success. I wanna expand my setup to fill a large garage with separate tanks but plumb them all into a sort of batch reactor setup similar to a water treatment plant just on a vastly smaller scale. Do you think I could get significant denitrification with this setup? Or would I get the same or better results running a heavily planted outdoor pond kind of like a sump tank?