
What follows is a guide to making a series of do-it-yourself (DIY) fertilizers for aquariums that are the preferred fertilizers for the author. The author prefers ammonium based fertilizers that put the iron and phosphorus into the plants in the form of root tabs. It is followed by some data on the chemicals for fertilizing aquariums. It is VERY long and boring and only meant for real aquarium nerds like the author.

The DIY Fertilizer Regimen I Recommend
I’m not exactly happy with the composition of ANY of the commercially available aquarium fertilizers. They do not add the nutrients where they should be added. They all add the nitrogen as nitrate. And they are all very expensive.
So I prefer using a homemade fertilizer that has the following characteristics:
- Adds phosphate and iron to the substrate, not the water column
- Uses ammonium rather than nitrate, depending on pH
- Is MUCH cheaper than the bottled fertilizers you buy on the internet

Algae do NOT have Roots
This addition of iron and phosphate fertilizer to only the substrate of an aquarium is based on a very simple truth: namely “Algae do NOT have roots!”. Rooted plants and algae are both plants. They both utilize fertilizers, carbon dioxide, and light to produce their growth. So rooted plants and algae are always in competition in a planted aquarium.
The obvious way to give rooted plants a competitive edge in this battle is to simply add the fertilizer to the substrate. By adding fertilizer only to the substrate ONLY rooted plants can access the fertilizer and thus ONLY the rooted plants can flourish.
Most (but not all) rooted “vascular” plants have been shown by testing to absorb phosphorus and iron from their roots much better than from their leaves. Most (but not all) vascular rooted plants also absorb potassium and nitrogen much better through their leaves.

Many add a “complete” soluble fertilizer to the water of a planted aquarium. This is a fertilizer that contains nitrogen, phosphorus, and potassium. While this can be done very successfully, especially with epiphytic plants or undergravel filters, one loses the potential of controlling algae by giving the rooted plants a necessary nutrient, phosphate, through the roots.
While potassium and nitrogen are so soluble that it makes little difference where they are added, phosphate typically has limited solubility in a fine substrate. One can SLOW DOWN but not STOP algae growth by adding phosphate only to the root zone of rooted plants.
Note this addition of phosphorus to the roots ONLY works with a fine substrate under 2 millimeter in size. A gravel substrate or an undergravel filter makes roots tabs useless. One might as well just add all the fertilizers to the water column. For fertilizer formulas which work with gravel substrates go to these articles:
15.5.3. Estimative Index
15.5.5. DIY Epiphytic Fertilizer

Homemade Fertilizer for Adding to the Water Column
I recommend a homemade water solution of nitrogen/potassium fertilizer added to the water column of most planted tanks.
A weekly cumulative added dose of nutrients for a high-tech aquarium with a moderate amount of lighting, CO2, and plants, according to this Homemade Fertilizer program, would be around:
- N nitrogen level of – 3.4 ppm (This is nitrate or NO3 of 15 ppm)
- K2O – Potassium – 12 ppm
- P2O5 – Phosphorus – added as root tabs
Or an “NPK” of 3.4-0-12 to the water column.

Two Component N/K Fertilizer
Potassium sulfate is the preferred salt for adding potassium for this type fertilizer. Unfortunately this potassium salt is relatively insoluble. So two solutions need to be made up. Each addition to the aquarium thus consists of two parts potassium solution to one part ammonium (nitrogen) solution.
Potassium Component
The potassium water-based fertilizer for the water column makes up a water solution made with one chemical obtained over the internet. Take one liter of hot distilled water and add:
- 123 grams potassium sulfate (0-0-55)
The potassium sulfate is very slow to dissolve. It may take a few hours and require reheating several times. Some crystals may be left in the solution. This solution is added at the same time as an addition of an ammonium salt solution.

Ammonium Component
The ammonium (nitrogen) water-based fertilizer for the water column makes up a water solution made with one chemical obtained over the internet. Take one liter of hot distilled water and add:
- 208 grams of ammonium sulfate (22-0-0)
“Macro” Fertilization
This creates roughly two separate 1 liters of “macro” liquid fertilizer with a composition of roughly 3.4-0-12 NPK, if two parts potassium is added directly to the aquarium for each part ammonium (nitrogen) added directly.
This water-based fertilizer is designed to be added every day first thing in the morning as TWO additions. Do not mix the two fertilizers as the salts will precipitate out. The dosages are shown in the charts below in this article. Note electronic dosing pumps are VERY useful to add this fertilizer. Do not store the fertilizer solution in the refrigerator as the chemicals will crystalize out of solution.

Ammonium is very rapidly consumed by stem plants, so it typically will be gone in one to two hours from any planted aquarium. And ammonia is vastly overrated as a fish toxin. So I use ammonium in planted aquariums.
Note that the timing of the dosing is important with fish in the tank. Add the fertilizer first thing in the morning, when the lights have been out for at least four hours. Note that ammonium is rapidly absorbed by stem plants even in the dark. And pH can rise rapidly in a good aquarium. So it is beneficial to have the lights come on one hour AFTER the fertilizer is added. But most hobbyists like to add both food and fertilizer to the tank first thing in the morning just after turning on the lights. This will be fine.
Do not add ammonium fertilizers after the lights have been on for any length of time. The pH of a planted tank can rise very high in a very short period and ammonium is poisonous at high pH. Note that timed dosing pumps are VERY useful for fertilizing any planted aquarium.

Flexibility
This fertilizer formulation is flexible. If one see new leaves with yellowing and the nitrate level of the water is below 20, add ammonium sulfate, potassium nitrate or urea. If one has holes in the leaves go ahead and add some potassium sulfate. If older leaves are turning yellow, add phosphate. Do not be afraid to experiment. Different plants respond differently to fertilizers and water chemistry plays a big factor.

Rigorous Fertilization Programs
Some hobbyists like to wing it and do their own thing when it comes to fertilization. Others want a rigid program that tells them exactly what to do. Either course of action is fine and can be made to work because Mother Nature is very flexible.
A “Complete” Fertilization Program for Low Tech Planted Aquariums
If the tank is low-tech without carbon dioxide injection add one homemade iron and two homemade phosphorus fertilizer tab under every rooted plant every five months (the recipe for these is below in this article). Then very frequently add the water-based fertilizer solution from above in this article.
Here is a useful chart for low-tech planted tanks for how much water-based potassium fertilizer solution to add to the water column every day, with the ammonium fertilizer being half this amount:
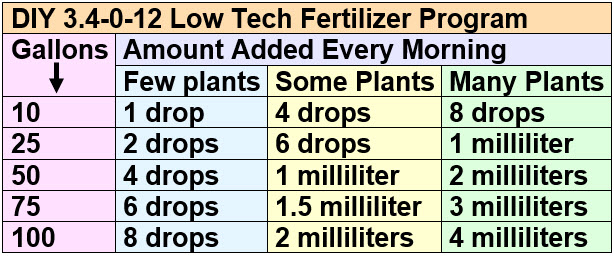
Again, take things slow and ramp up. Plants normally don’t absorb fertilizers very well for the first few months. Low-tech planted aquariums require patience. Note that the more frequently one adds this soluble nitrogen and potassium fertilizer the better the results. I used a dosing pump that added a dose one hour before the lights came on every single day.
Some useful conversions are:
- 1 tablespoon = 3 teaspoons =15 milliliters
- 1 teaspoon = 5 milliliters
- 1 milliliter = twenty drops

A “Complete” Fertilization Program for High Tech Planted Aquariums
For a rigid program for a high-tech CO2 injected high light planted aquarium put one DIY iron tablet and two DIY phosphorus tablets per rooted plant deep into the substrate once every month (the recipe for these tabs is down below in this article). Then add the water-based fertilizer solution from above in this article once daily first thing in the morning:
Here is a useful chart as to the amount of water-based potassium fertilizer solution to add once a day to a high-tech planted aquarium, with the ammonium based fertilizer being half that dosage:
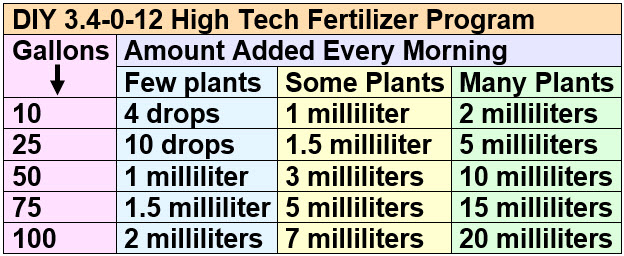
If one has fish in the aquarium, add the water-based fertilizer solution after at least four hours of dark in the aquarium. This avoids any possibility of ammonium toxicity. Dosing pumps are very useful for making these additions. If you use a dosing pump add the fertilizer after at least four hours of dark in the aquarium but one hour before the lights come on in the aquarium. This maximizes absorption by the plants. Note that most hand sanitizer bottles dispense one milliliter of solution per push.

Again, the amount of fertilizer, carbon dioxide, plant mass, and light in a high-tech tank need to be “balanced” based on plant mass. If one has just started a planted aquarium with a few clumps of stem plants, use 10% of the fertilizer amount, 10 ppm CO2 and 30% lights on for 6 hours. If one has a very well-planted tank with a large mass of well-established stem plants, double the fertilizer amount, keep the CO2 at 30 ppm, and keep high-intensity grow lights at 100% on for 16 hours.
Note that the more frequently one adds this soluble nitrogen and potassium fertilizer the better the results. I used a dosing pump that added two doses (one ammonium, one potassium) of water-based fertilizer solution one hour before the lights came on every single day.
Some useful conversions are:
- 1 tablespoon = 3 teaspoons =15 milliliters
- 1 teaspoon = 5 milliliters = 100 drops
- 1 milliliter = 20 drops

Homemade Root Tabs
Plant tabs are small capsules that are inserted into the substrate to deliver the fertilizer rooted plants need directly to the roots. This will NOT stop algae growth in the aquarium. Algae can grow at a VERY LOW concentration of nutrients. Green spot algae and diatoms can grow at fifty parts per billion of nutrients. That is 0.050 parts per million. But the growth will be slow and plants growing at a decent rate can control this algae with chemicals (negative allelopathy).
One can easily make phosphorus plant root tabs using the following formula:
- 75% Plaster of Paris (calcium sulfate or gypsum) binder
- 15% Epsom Salts (magnesium sulfate)
- 10% Triple Phosphate (other names are “superphosphate”, superphosphates, and calcium dihydrogen phosphate), available from the internet. While the calcium hydrogen phosphates are preferred here, this can be any phosphate salt, including dipotassium phosphate, monoammonium phosphate, and diammonium phosphate. Note avoid bone meal (apatite) as it doesn’t decompose very well in the aquarium. Note I found all these phosphate chemicals difficult to find in some countries.
Note I don’t pay a lot of attention to the exact mix. Very roughly two tablespoons of Plaster of Paris, one teaspoon of Epsom salts, and two-thirds teaspoon of phosphate are just fine. This isn’t rocket science. Also, note that the Plaster of Paris (calcium sulfate or gypsum) is not a fertilizer. It is only a binder that slowly dissolves and releases the phosphate.

One can easily make iron plant root tabs using the following formula:
- 80% Plaster of Paris (calcium sulfate or gypsum) binder
- 15% Epsom Salts (magnesium sulfate)
- 3% ground pyrite (iron sulfide, internet purchase) or finely shredded steel wool. This addition is optional.
- 2% ferrous sulfate powder or iron chelate (garden store or internet). Any type of ferrous sulfate or iron chelate is fine. Ferrous sulfate is also known as iron sulfate, green copperas, and green vitriol (caution, blue copperas and blue vitriol are copper compounds). And any iron chelate is fine.
Note I don’t pay a lot of attention to the exact mix. Very roughly two tablespoons of Plaster of Paris, one teaspoon of Epsom salts, a one-fourth teaspoon of pyrite or steel wool, and a one-sixth teaspoon of ferrous sulfate or chelated iron are just fine. This isn’t rocket science. Also, note that the Plaster of Paris (calcium sulfate or gypsum) is not a fertilizer. It is only a binder that slowly dissolves and releases the iron.
The reason to make up two separate tabs is that phosphorus and iron tend to cancel each other out in a fertilizer. In a well-oxygenated environment, especially at high pH, the iron phosphates which form are very insoluble.
Note these tabs will not work in gravel. Large diameter substrates and under-gravel filters have such a large exchange rate with the water column that tabs do little. The nutrients enter the water column almost as soon as they leach out of the tabs.

Note that triple phosphate, pyrite, and plaster of Paris are natural products that have all the micronutrients (save iron) necessary for plant growth. So adding a micronutrient supplement from the garden store is NOT warranted. But if it makes one “warm and fuzzy” add just a pinch of a garden micronutrient mix.
Many are concerned about micronutrient toxicity. These concerns are not warranted by the math. For instance, a level of 10 parts per million of manganese becomes toxic to some plants (“The Physiology of Manganese Toxicity”, Horst, W.J. 1988).
The manganese level in Flourish Trace™ is 85 parts per million in the bottle (8.5 x 10-8). The directions are “Use 1 capful (5 mL) for every 80 L (20 US gallons) twice a week“. The calculations are 5 ml is 0.0013 gallons. 0.0013/20 = 0.000065 (6.5 x 10-5). then (8.5 x 10-8) X (6.5 x 10-5) = 55.25 x 10-13 . This is a concentration of manganese in the aquarium water of 0.0000055 parts per million per application. The math become 10 divided by 0.0000055, which is 200,000. So to get to 10 parts per million one would need to dose 200,000 times without a water change. Not exactly something to worry about.
The same math applies to using products such as a garden micronutrient mix. The levels of micronutrients are just too small to be a concern.

Mix the ingredients for both tabs thoroughly, adding just enough water to make a very thick putty (the consistency is that of a drywall compound). Put it into the smallest gelatin capsules one can buy on the internet. These are size 4 gelatin capsules. The filling operation is not easy. The tabs are small and difficult to fill.
Note that these gelatin tabs are composed of animal protein (cooked down hides, hooves, and hair). If you have fish much over two inches in size these fish will smell the gelatin and uproot the tabs. So, if you have larger fish, it is best to make a thin slab of the Plaster of Paris mix and just cut it up into small cubes before it hardens. Indeed, if you don’t want to go through the pain in the butt process of filling the tabs, just do the slab and cut method.

Note that ground pyrite is interesting. It is a mineral (“fool’s gold”) that is attacked by certain bacteria. The bacteria slowly break it down into ferrous sulfate. This slow breakdown feeds the plants the tiny amounts of iron they need constantly. But it isn’t available in some countries and it can simply be skipped with little impact. A good alternative is finely shredded steel wool.
Push the tabs as deep into the substrate as you can. The rate at which the tabs dissolves depends on three elements: the depth they are placed at, the particle size of the substrate, and the total dissolved solids TDS) of the water. The deeper the tabs are placed, the smaller the substrate particles, and the higher the TDS the slower they will dissolve. They might dissolve in a few weeks in shallow placement in large grained sand substrate and soft water. They might take six months two inches deep in a fine sand substrate with hard or salty water.

When providing plants with calcium it is always important to include some magnesium (3 parts calcium to 1 part magnesium is ideal but the above formula doesn’t work too well at this ratio). Calcium and magnesium compete against each other in plant uptake so supplying calcium without magnesium is always a touchy thing to do. Magnesium is a necessary ingredient in chlorophyll, which is important to all plants.
The calcium will slowly make the water hard. We do recommend water changes of 25% once a month to 50% once every two months in any aquarium with light fish stocking. These water changes will keep this hardness buildup controlled.

Other Plant Tabs
Some E-bay entrepreneurs put garden Osmocote™ products into gel caps and sell them as aquarium fertilizers at ridiculous markups. If you want to go that way, buy both the gel caps and Osmocote™ and make your own. These Osmocote™ tabs are a very good choice for placing deep into the substrate under the plant if you do not want to go through the hassle of the plaster of Paris tabs.

Note that these gelatin tabs are composed of animal protein (cooked down hides, hooves, and hair). If you have fish much over two inches in size these fish will smell the gelatin and uproot the tabs. So, if you have larger fish, it is best to make a slab of Osmocote™ in Plaster of Paris and just cut it up into small cubes before it hardens.
Also, note that there are pills for both iron and phosphates sold as “supplements” for human consumption in Walmart. The phosphate pills are very good low-cost options for putting into a fine substrate in a planted aquarium. They are much cheaper than commercial aquarium plant tabs. But the iron tablets have too much iron in them. Do not use iron tablets.

A Note of Caution
Here is a comment from one of my readers:
“I also attempted to make the homemade fertilizer using the second formula with urea, ammonium sulfate, and potassium sulfate. I’m not sure if I should mix it into the water first then add it to the tank, and I’m not sure how much to use for a 10-gallon planted tank that hasn’t matured yet. It’s been about 3 months and I’ve only been using Flourish tabs up until everything came in 3 days ago. only about a quarter of the substrate is planted and there are some floating plants, so I used a cereal spoon and just added 3 scoops. one on the left side, one in the middle, and one on the right side. A couple of days later I woke up to a bacterial bloom. one of the fish that died was a black molly but it had red sores all over it that weren’t there yesterday, and the clown pleco decided to float at the top upside down. It’s still alive because it will randomly take off to another part of the tank every so often, but I’ve never seen it do this before. it also looks slightly bloated, but I’ve only seen it on the glass or some plants, so I don’t know if it’s normal or if it’s new. She has gotten wider as she got older, but she looks bloated in the stomach area, not the sides. on top of this, the whole tank has an awful smell that wasn’t there yesterday. A week ago it had a very weak smell, and I had to be very close to it to notice it was there. Now it’s like a sweaty gym bag. Is it possible I added too much ammonium or that there wasn’t enough CO2 in the water for the plants to use everything so the bacteria got it first? or do you think this was an issue that was building over the last week or so because of something else?”
I felt horrible when I got this message. He added “three scoops” to a ten-gallon tank?? I did not explain things well enough, especially if by “scoops” he means dry fertilizer salts. But even if he only added three teaspoons of the solution he added easily 38 times the dosage for a very well-planted aquarium and 380 times the dosage for a sparsely planted aquarium. And he killed his fish, which makes me feel very guilty. When I got this I rewrote this article about three times, hoping to prevent such a disaster by making this easier to understand with clearer instructions.

The Math for Soluble Fertilizers for the Water Column
The math for the ammonium sulfate is:
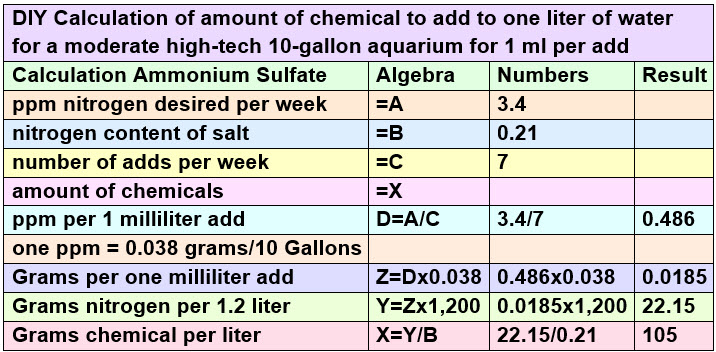
.

These are the water-based fertilizer solution calculations for the 0-0-60 potassium sulfate:
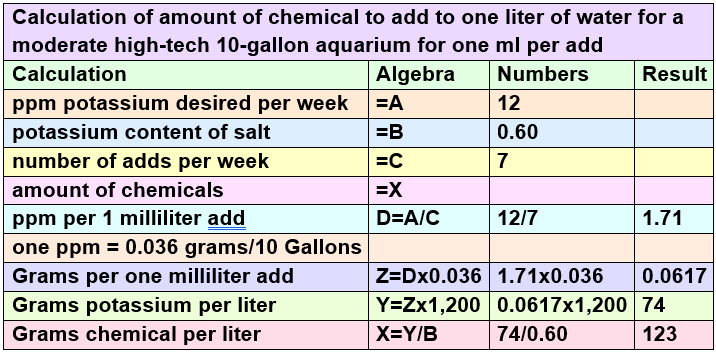
Note ammonia salts all have problems:
- ANY ammonium salt will precipitate potassium sulfate out of solution.
- Ammonium bicarbonate and carbonate will considerably raise the pH with each addition
- Ammonium chloride can produce chloride toxicity in plants
- Ammonium nitrate is only 50% ammonium.
So ammonium sulfate becomes the lesser of the evils. Note that small quantities of ammonium sulfate can be ridiculously expensive. It is much cheaper to buy 20 to 50 pound bags of ammonium sulfate from an agricultural fertilizer store.
One needs to monitor pH when using this formula. The pH should not go above 8.2 first thing in the morning in a planted tank with fish in it. If the pH is above 8.2 pH after the lights have been out for at least eight hours one must go over to the following for the nitrogen/potassium fertilizer:
- 340 grams potassium nitrate (NPK 13-0-44)
This removes the advantage of the ammonium but retains the advantage of the root tabs. This gives a fertilizer solution of 3.3-0-11.2.
Repeating myself, it should be noted tabs only work with substrate less than 2 millimeter in diameter. Gravel or an under-gravel filter will leach the tab nutrients into the water so fast that there is no benefit to the tabs.
An Easy Alternative
An easy way to regulate all this math is to simply pick the desired nitrate level and add fertilizer accordingly. For instance, measure the nitrate level once a week. If the nitrate level is above 20 ppm, do not add fertilizer for a week. If the nitrate is less than 20 ppm, add fertilizer per one of the schedules above. Easy! Or try 10 or 40 ppm. Some even go for 80 ppm.

Other Fertilization Programs for High Tech Planted Aquariums
It is useful to compare the above fertilization program with what various others recommend. Here are three common methods of fertilization.
Probably the most popular method of fertilization of high-tech planted aquariums is the EI method, originated by Dr. Tom Barr. For most planted aquariums Dr. Barr recommends nitrate nitrogen fertilizer at a level of 3.4 nitrogen, 1 ppm phosphate (P2O5), and 12 ppm potassium (K2O), NPK of 3.4-1-12, per week. For a low-tech tank or low plant mass tank Dr. Barr recommends the levels be brought down to only 20% of this level (0.68 ppm nitrogen, 0.2 ppm phosphate, 2.4 ppm potassium per week). Dr. Barr says the high-tech level can even be doubled for an aquarium with a very large mass of thriving plants.
Aquarium Co-Op Easy Green™ (NPK of 2.7-0.5-9) uses nitrate nitrogen for a total addition per week to a low-tech aquarium of 0.7 ppm nitrogen per week, 0.1 ppm of phosphate, and 2.3 ppm potassium. Easy Green doesn’t have a dosage for high-tech tanks but if one multiplies by five one gets 3.5 ppm nitrogen per week, 0.5 ppm of phosphate, and 11.5 ppm potassium.

For a high-tech planted aquarium with CO2 injection and high lighting levels, some hobbyists measure the nitrate level once a week. If the nitrate level is below 20 they add 10 ppm (0.38 grams x 10 per 100 gallons = 0.38 grams per ten gallons) of nitrate or roughly 0.65 grams per ten gallons of potassium nitrate (NPK of 13.7-0-46). If the nitrate level is above 20 they add 0.65 grams per ten gallons of a potassium salt other than nitrate. This is the equivalent of 2.8 ppm nitrogen and 6.6 ppm potassium added once a week. They depend on fish food to supply phosphorus and iron.
All three of these methods work just fine. But there is considerable research out there that says ammonium is twice as good as urea and six times better than nitrate when growing submerged plants. So I much prefer ammonium fertilization. And I much prefer to put my iron and phosphate into the substrate.
Research has shown a level of potassium roughly three times greater than nitrogen is preferred by vascular plants growing in water and all these fertilizer programs reflect that research. The homemade fertilizer above has nitrogen and potassium levels that are very close to what all these other programs recommend.

Calibration for Fertilizer
Adjusting the water column fertilizer amounts in an aquarium is pretty easy. One wants to add sufficient ammonium that roughly 0.5 ppm ammonium is measured two hours after adding the ammonium fertilizer. If it is more than 0.5 ppm, drop the amount of the fertilizer added at the next scheduled addition. If it is below 0.5 ppm, add to the amount of fertilizer added at the next scheduled addition.
Soft Water
There is one situation where a fertilizer of sorts is called for in all types of fertilization. If the water is very soft (GH less than 3 or dGH less than 72) then it needs both calcium and magnesium additions for most plants. The easiest way to do this is to simply add one level teaspoon of Epsom salts (magnesium sulfate) and one level teaspoon of Plaster of Paris (calcium sulfate and calcium carbonate) to every ten gallons of water change water.

The Science behind Fertilizer Chemicals
The “NPK analysis” found in all fertilizers is a series of three numbers (like 13-3-15) which indicates the percentages of nutrients in the fertilizer. The first number is nitrogen (N). The second number is phosphorus (P or actually P2O5). And the third number (K or K2O) is potassium (“K” is from the Latin word for potassium – kalium).
Here are the chemicals we are using above:
.

Ammonium sulfate is the chemical formula (NH4)2SO4. It has an NPK analysis of 21-0-0
.

“Triple Phosphate” or “Super Phosphate”. This chemically is calcium dihydrogen phosphate or monocalcium phosphate, [Ca(H₂PO₄)₂ .H₂O]. The NPK analysis is 0-46-0
.

Potassium sulfate is K2SO4. It has an NPK of 0-0-55
.
Potassium nitrate is KNO3. It has an NPK of 13-0-44
.

Ferrous sulfate is FeSO4 or “Iron sulfate” and is used to supply iron to the planted aquarium.
.
.
Pyrite is useful for supplying iron in very slow amounts to plants. It is made for use in jewelry.
.

Epsom Salts are just magnesium sulfate. It is available in any drugstore for making a solution to soak feet. Magnesium is normally available in most waters at adequate levels but needs to be added if one is adding calcium.
.
.

Plaster of Paris is chemically just Gypsum (CaSO4-2H2O) which has had 75% of the water driven out. It typically has up to 40% calcium carbonate (ground limestone) in it as a cheap filler. When water is added to the powder the plaster becomes a hard solid material. It is widely used in arts and crafts hobbies. It is ONLY used here as a binder, it is not a fertilizer per se.
Fertilizers in More Depth
We go into aquarium fertilizers in more depth in the following links:
15.5. Aquarium Fertilizing
15.5.1. Ready-Made Fertilizer
15.5.2. Fertilizer Programs
15.5.3. Estimative Index
15.5.5. DIY Epiphytic Fertilizer
15.5.6 Fish Food as Fertilizer
15.5.7. DIY Fertilization
.
Return to Planted Aquarium Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit-making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Dave says
In reply to Kajetan ….. Ferrous sulfate readily precipitates out as ferrous hydroxide (yellow color) in water. Nothing to worry about save to use vinegar instead of water. Even vinegar will precipitate out though. There is still plenty of “free” ferrous ions in the solution. So either don’t worry about it or use chelated iron.
Kajetan says
Thank you so much for the answer.
One more thing:
Wouldn’t the ferrous sulfate powder oxidise in the making of the fertiliser capsules?
I just made both types of caps and I can see the Fe ones have gotten a yellowish hue to them. Isn’t that just the ferrous sulfate powder oxidising? Maybe I shouldn’t have disolved the ferrous sulfate powder in the mix but added it as crystals just before hardening?
Regards
Kajetan
Dave says
In reply to Kajetan ….. Monopotassium phosphate should be just fine
Kajetan says
Dave I have a quick question if you would be so kind as to help me.
Could I use Monopotassium phosphate (KH2PO4) in my DIY root tabs? I think it should be fine but a second opinion would help me a lot.
Thank you.
Sasha says
Hello, David!
Thank you for your amazing articles, they’re very informative and reduce my newbee anxiety a lot.
I have 2 questions about epsom salts in your recipe:
1. It’s not a common term where I live, so I did some digging on Wikipedia to be sure. The article says that it’s not just MgSO4, but MgSO4•7H2O (magnesium sulfate heptahydrate), which seems very important, because this makes 53% of its volume – water and only 47% magnesium sulfate, unlike, for example, a fertilizer that is just “dry” salt (my favorite source is a video by a very cool chemist NileRed, where he shows it in practice: https://youtu.be/8GVSuKkuLzY?si=bmdsCs8kK10VysiW). So, which do you mean by saying “15% Epsom Salts (magnesium sulfate)” in your recipe? Even some products that state to be “epsom salt” have “99% MgSO4” and nothing else in contents and look suspiciously like powder, not crystals.
2. My mom has a bag of that same suspicious powdery “epsom salt” and on the back it promises to be “a source of essential elements in the form of S, Mg and an admixture [trace amount, impurity – not sure how to translate] of Cu”… @&£*%!! I have shrimp and this worries me. Is this a common statement for epsom salts? Is it worthy of consideration?
And perhaps a remark about a very similar thing with gypsum and plaster of Paris: these are CaSO4•2H2O and CaSO4•0,5H2O respectively. You do state it later in your article, but not in your ingredients list. When it comes to preparation, one (me) would return to the article just for the ingredients list, where it looks like you can use the former interchangeably with the latter and drywall, no adjustments needed. Especially for dense folks like I seem to be today, after a couple hours of looking up what equivalents does my country have for the products you mention. And I had an F for chemistry in school, I don’t know how to compare the volume of a water molecule to that of CaSO4. After the epsom salt situation it seems to be more complicated than just looking at numbers in the formulas. So, since the amount of Ca is important for the Mg adjustment, I think, it would be better to also state how much of gypsum one should use in the ingredients list. And I’ll just use plaster of Paris (which is commonly called GYPSUM in my country – aaarrgghhh).
Anonymous says
Hello, David!
Thank you for your amazing articles, they’re very informative and reduce my newbee anxiety a lot.
I have 2 questions about epsom salts in your recipe:
1. It’s not a common term where I live, so I did some digging on Wikipedia to be sure. The article says that it’s not just MgSO4, but MgSO4•7H2O (magnesium sulfate heptahydrate), which seems very important, because this makes 53% of its volume – water and only 47% magnesium sulfate, unlike, for example, a fertilizer that is just “dry” salt (my favorite source is a video by a very cool chemist NileRed, where he shows it in practice: https://youtu.be/8GVSuKkuLzY?si=bmdsCs8kK10VysiW). So, which do you mean by saying “15% Epsom Salts (magnesium sulfate)” in your recipe? Even some products that state to be “epsom salt” have “99% MgSO4” and nothing else in contents and look suspiciously like powder, not crystals.
2. My mom has a bag of that same suspicious “epsom salt” and on the back it promises to be “a source of essential elements in the form of S, Mg and an admixture [trace amount, impurity – not sure how to translate] of Cu”… @&£*%!! I have shrimp and this worries me. Is this a common statement for epsom salts? Is it worthy of consideration?
And perhaps a remark about a very similar thing with gypsum and plaster of Paris: they are
Dave says
In reply to Andrew …. “Human” iron supplements are not timed release and are available as soon as the pellet dissolves (a few hours at most). “My formula” root tabs are encased in a slow release Plaster of Paris formula which should take weeks to months to dissolve and release the iron sulfate. So the caution remains not to use “human” supplements for iron.
Andrew Snider says
Hello,
I had a question on your root tabs. You talked about human supplements as root tabs. Iron supplements have 325mg ferrous sulfate, and phosphate have about 600mg per the labels. You said the Iron pills had too much for aquariums?
Your formula I’m estimating 1/6 teaspoon ferrous sulfate as being about 4 grams of ferrous sulfate, and 2/3 teaspoon of phosphate as being about 12 grams of phosphate.
Did I screw something up? Or do you think maybe I could get away with the supplement route?
Thank you, love your site, Andrew
Zach says
I just wanted to point out that it may be worth mentioning that aquarists should avoid using standard potash that is used for landscaping — this is typically known as “muriate of potash”. Although the ingredients on the bag will say “K2O”, it is actually KCl. This means adding muriate of potash will add one chloride ion for every potassium ion (not ideal!). The header at the top of the page actually has a photo of muriate of potash, but luckily the article does not mention it. I just wanted to point this out because I almost fell into this trap myself in the past.
Dave says
In reply to Frazer …. I don’t know. I can’t find any information on Neo Plants by Aquario. As for your melting plants that is quite normal. The plants you buy are grown emergent (i.e. in air). When emersed in the aquarium these leaves die and are replaced with leaves that will grow under water.
Frazer R Dougal says
Hi very informative and I am going to research this a bit more before I jump in. I have a question though. At the moment I have been using these Neo tablets by Aquario. Neo Plants they are called. Almost like like pelletized wood. Either way they look decent enough and arent not expensive at all but what I am finding (and I have emailed this company about this) the tablets are turning black and getting a bad rot smell to them. Like rotting plants. Some of them not all are doing this and I am not sure why. I have alot of plants and the base int he tank is about 1 inch of flourite sand and on top is about 1 to 2 inches of natural sand. Just about all my plants for some reason go thru a melt when purchased then just before they all die they spring to life and go nuts. MOST anyways. But why are these root tabs doing this. Is it the SAND….or is this normal. I am frustrated to the point thinking my choice of sand was a mistake and thinking maybe I should pull most and add a coarser material like more flourite or stratum. Any advice would be appreciated and also why you think these tabs are turning black and getting a bad rot smell to them.
Dave says
In reply to Jose… baking clay in a standard oven gives a very porous tablet that will slowly leach out the phosphate
Jose says
Love reading through your articles, all super informative. A question about DIY root tabs: I want to make my own to dose phosphate into soil, but have noticed that anything with plaster of Paris really raises my Gh, and I’m trying to keep parameters stable for my shrimp. I have heard that one can use red sculpting clay as a binder, mixed with dry ferts and baked. But others say they find the clay tabs still present months after, undissolved. However I’m unsure whether this would hinder the clay from releasing its nutrients. Any experience/thoughts on this? I also have this crazy idea to just use some broken up aqua soil as a binder.