
Rooted Plants versus Epiphytes and Floating Plants
Some planted aquariums have epiphytes (plants that grow on wood or rock) and floating plants. Tabs put into the substrate obviously won’t work too well for these epiphytes as they do not have roots into the substrate. So they have to be supplied phosphate and iron through the water column. This means the fertilizer recipe preferred by the author needs to be amended.
This method is also useful with gravel substrates, including under-gravel filters as root tabs don’t work with gravel substrates.
The fertilization program then becomes similar to the Estimative Index method of fertilization save for one difference:
- the use of ammonium rather than nitrate to supply the nitrogen in aquariums with a pH less than 8.2

A weekly cumulative added dose of nutrients high-tech tech aquarium with a moderate amount of lighting, CO2, and plants would still be around:
- N nitrogen level of – 3.4 ppm three times per week
- P2O5 Phosphate – 1 ppm three times per week
- K2O – Potassium – 12 ppm three times per week
- Fe/traces – Iron and micronutrients – 0.2 ppm three times per week (alternating with the three ingredients above)
Three fertilizer formulations are needed. The potassium component (macro #1), the ammonium/phosphorus component (macro #2) and the one with iron (the “micro”).

Two Component N/K Fertilizer
Potassium sulfate is the preferred salt for adding potassium for this type fertilizer. Unfortunately this potassium salt is relatively insoluble. So two solutions need to be made up. Each addition to the aquarium thus consists of two parts potassium to one part ammonium (nitrogen).
The potassium water-based fertilizer for the water column makes up a water solution made with one chemical obtained over the internet. Take one liter of hot distilled water and add:
- 123 grams potassium sulfate (0-0-55)
The potassium sulfate is very slow to dissolve. It may take a few hours and require reheating several times.

The ammonium (nitrogen) and phosphate water-based fertilizer for the water column makes up a water solution made with two chemicals obtained over the internet. Take one liter of hot distilled water and add:
- 197 grams of ammonium sulfate (54-0-0)
- 35 grams diammonium phosphate (18-46-0)
This creates roughly two separate 1 liters of liquid fertilizer with a composition of roughly 3.4-1-12 NPK, if two parts potassium is added directly to the aquarium for each part ammonium (nitrogen) added directly.
This water-based fertilizer is designed to be added every day first thing in the morning as TWO additions. Do not mix the two fertilizers as the salts will precipitate out. The dosages are shown in the charts below in this article. Note electronic dosing pumps are VERY useful to add this fertilizer.
Note that phosphates form a compound called “polyphosphate” which can form a filmy cloudy kind of mulm at the bottom in a bottle of fertilizer. Just ignore it. Do not store the fertilizer solution in the refrigerator as the chemicals will crystalize out of solution.

Several other ways to make such a fertilizer are covered in this link:
15.5.7. DIY Fertilization
Flexibility
These fertilizer formulations are flexible. If one see new leaves with yellowing and the nitrate level of the water is below 20, add potassium nitrate or ammonium nitrate. If one has holes in the leaves go ahead and add some potassium sulfate. If older leaves are turning yellow, add phosphate. Do not be afraid to experiment. Different plants respond differently to fertilizers and water chemistry plays a big factor.

“Micro” Fertilizer for Epiphytic Plants
In addition, for a high-tech epiphytic aquarium, mix up 750 milliliters of distilled water and 250 milliliters of vinegar with 20.5 grams of iron sulfate (ferrous sulfate heptahydrate which is 20% iron by weight). One can also add 3 to 6 grams of garden store micronutrient mix to the vinegar.
The use of micronutrients other than iron is somewhat controversial. I can find no research which says the micronutrients (other than iron) are necessary with a normal water supply. Nor can I find any research which says a moderate amount of micronutrients do any damage.
Add one milliliter of this iron (plus micronutrients if desired) solution per 10 gallons three times a week for a moderately planted aquarium with moderate CO2 and moderate lighting. Alternate this iron fertilizer with the above phosphate-containing fertilizer so that iron and phosphate are not added at the same time.
Note that one can increase the iron concentration and simply proportionately reduce the amount put in. For instance, if one has a moderate 50-gallon tank one can take the ferrous sulfate addition to 60 grams in a liter (12×5=60) and add one milliliter for the 50 gallons.

One can also use any solution of acid in distilled water with a pH of 5 to 6 to stabilize the ferrous sulfate. This can be weak solutions of hydrochloric acid (“pool acid”) or any other mineral acid. Adding this acid can take the KH of the aquarium to zero but that is not a problem as long as the pH first thing in the morning is above about 6.0. And even then a pH below 6.0 will only be a problem with fish in the tank as nitrites can become somewhat poisonous at that low of a pH.
Some with very soft water (less than 4 GH) add 60 grams Epsom Salts and 40 grams calcium acetate to the ferrous sulfate solution to give the plants the calcium and the magnesium they require. Note I avoid using calcium chloride here as I’m not a big fan of mixing plants and unnecessary chlorides. A bag of crushed coral in the filter is ALWAYS a good idea.

“Macro” Fertilizer Above 8.2 pH
As mentioned above, if one has fish in the tank one has to be aware of potential ammonia toxicity. The formulas above are fine for tanks up to 8.2 pH (measured first thing in the morning before the lights are turned on). Above 8.2 pH just go to the formula for the EI methodology:
- 1 liter heated water
- 328 grams potassium nitrate (NPK 13-0-44)
- 16 grams mono-potassium di-hydrogen phosphate (NPK 0-52-34)
First, dissolve the potassium phosphate into the hot water and then dissolve the potassium nitrate. This creates 1 liter of liquid fertilizer with a composition of roughly 3-0.6-11 NPK. This formula only requires half the amounts in the graphs below as it is twice as strong.

Schedule for Epiphytic Aquarium Fertilizer Formulas
Since one is supplying the iron and phosphate in the water column rather than in the substrate the schedule for fertilizing plants becomes the same as the EI method:
- Day one, do nothing
- Day 2 add N-P-K (nitrogen, phosphate, and potassium) fertilizer to the water column
- Day 3 add iron fertilizer to the water column
- Day 4 add N-P-K (nitrogen, phosphate, and potassium) fertilizer to the water column
- Day 5 add iron fertilizer to the water column
- Day 6 add N-P-K (nitrogen, phosphate, and potassium) fertilizer to the water column
- Day 7 add iron fertilizer to water column fertilizer
It is important to add this fertilizer ONLY after at least four hours of darkness in the aquarium. First thing in the morning is always the best. If the lights have been on for any length of time the CO2 level can easily drop to the point where the pH goes over 8.2. Ammonium will become very stressful to fish at any level above 8.2.

This is the amount of potassium and iron fertilizers to add per addition. The amount of ammonium (nitrogen) is half this per addition since the potassium isn’t very soluble and can only be made up in half concentrations.
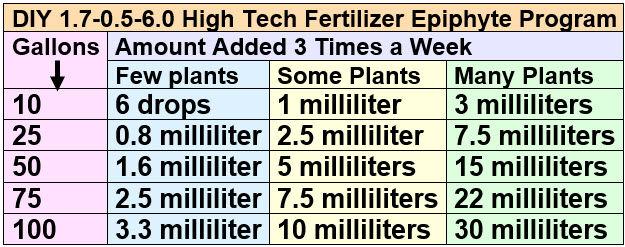
.
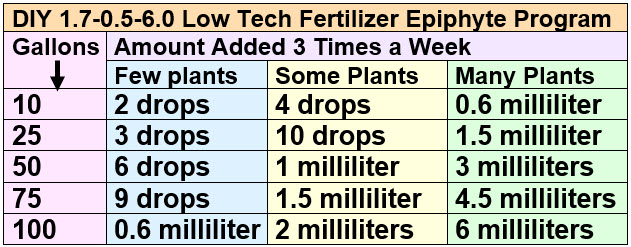
An alternative method of dosing is to measure the nitrate level the night of a day where the micros (iron) is added. If the nitrate level is below 40 ppm, add more fertilizer. If the nitrate level is above 40 ppm, decrease the amount of fertilizer being added. Note some aim for 5 ppm nitrate, others aim for 80 ppm. I like 40 ppm nitrate.

Fertilizers in More Depth
We go into aquarium fertilizers in more depth in the following links:
15.5. Aquarium Fertilizing
15.5.1. Ready-Made Fertilizer
15.5.2. Fertilizer Programs
15.5.3. Estimative Index
15.5.4. NH4 + Tabs Fertilizer
15.5.6 Fish Food as Fertilizer
15.5.7. DIY Fertilization
.
Return to Planted Aquarium Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit-making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Mik says
Thanks for confirming no need to refrigerate, have a great day!
Dave says
In reply to Mic…… You are correct. This is an update. There is no need to refrigerate. Good catch
Mik says
OK, I found it! It’s on your EI page (https://aquariumscience.org/index.php/15-5-3-estimative-index/). Same exact formula of 750ml distilled water, 250ml vinegar, 20.5g ferrous sulfate heptahydrate. But on the EI page under the “iron plus micro nutrients” section it says you must refrigerate it. I must admit, before I realized you said to refrigerate, I didn’t and I used the solution weekly for about 6 months. It was in an opaque container, not sure if it ever grew anything.
I guess I’m asking if you’ve updated your methodology on refrigerate or not.
Mik says
Hi again! I use your formula for iron supplements and I thought I recall you recommending the vinegar /iron mix to be refrigerated, but I can’t find it anymore. I’ve noticed you’ve been doing updates throughout your site so I’m wondering if this is an update.
Thanks!
Dave says
In reply to Rahul… The nitrogen in the ammonium sulfate is only 22% (my screw up). But note I used 22% in the calculations. NPK is based on weight so they are accurate, even though the 1.4 liters in inaccurate. Note the NPK numbers are the published NPK for the compounds, which take into account impurities. For instance ammonium phosphate fertilizer grade has significant amounts of sulfate in it.
Dave says
Good catch on the lack of expansion due to adding salt. I missed it completely. I have corrected the narrative to reflect weight, not volume. The schedules for low tech and high tech are the same as found in the article on DIY Fertilizers. And normally three parts calcium to one part magnesium is recommended. Because of waters of hydration it comes out to VERY ROUGHLY equal parts Epsom salts and Plaster of Paris. And you are correct GH only gives you the sum of the calcium and the magnesium. And yes, the charts are based on 20 drops in a milliliter.
Rahul says
Also, dissolving these salts in water , probably will not result in such a volume change..
Like dissolving 328gm of kno3 and 16gm of khpo4 in 1 liter of water will not make it 1.4 liter of solution..the total weight will be approx 1.4kg yes, but it will be only slightly above 1 liter..
It will just be a denser solution..
In that case, the calculations per ml of fert added will change so , it probably needs to be reviewed in these articles..
Can you kindly mention the basic ppm of N,P,K that needs to be added for low tech /moderate/hi tech tanks and their schedule for normal planted tanks ( similar to the chart provided for epiphytes)
The rest of the calculation then can be made accordingly ..
Also, can you also give the recommended calcium : magnesium ratio
And total cal+ mag ppm recommended?
I take it that gh calculation can give a rough estimate of total ca+mg content in the water ( but not the ratio), is that true?
Also, in these calculations, i think 20 drops make an mL?
Rahul says
I have some problem in understanding the concentrations..
Here, ammonium sulphate is 55 percent N
So 197 gm will give 108.3 gm N
Diammonium phosphate is 18 percent N and 46 percent P (as p205) gives
6.3gm N and 16.1 gm P
So total they give 114.6 N : 16.1 P
For k2so4
It’s 55 percent k2o
So 123 gm gives 67.65 k2o
Twice this gives 135.3 k
Combining this we get a ratio NPK of
108.3: 16.1: 135.3
Which comes down to 7:1:8.4 and not 3.4:1:12
Also, molecular weight of ammonium sulphate and DAP are both 132
N being 14 and two molecules of N being 28
The percentage N in ammonium phosphate is 21 and not 55
And percentage N in DAP is 21 and not 18 ( but close at least)
Also percentage of P (as p2o5) is 54 and not 46
( In other compounds like k2so4 and kno3, khpo4 this calculation and ratio holds true..but not in these ammonium salts..
And even if I calculate NPK with the calculated percentage of 21 and 54
The final ratio comes at 2.6:1:7 which is also not 3.4:1:12 closer but nowhere near..
What am I missing?