
There are several methods of fertilizing a planted aquarium:
- The easiest approach to planted aquarium fertilizing is to simply use a “ready-made” fertilizer like Aquarium Co-Op Easy Green™ per the instructions on the container. Easy. Simple. Effective. But this method is not cheap.
- Adding an expensive complete commercial liquid fertilizer “program” to the water column with multiple expensive bottle of various and sundry “fertilizers”.
- Adding inexpensive, simple DIY nitrate-nitrogen, phosphorus, and potassium fertilizer to the water column
- The inexpensive DIY Estimative Index methodology of Dr. Tom Barr (This labor-intensive method is probably the most popular method among the “experts”)
- Adding inexpensive DIY ammoniacal nitrogen and potassium to the water column with phosphorus and iron tabs to a fine substrate (my preferred method)
- Adding inexpensive DIY ammonia nitrogen, phosphorus, potassium, and iron epiphytic fertilizers to the water column
- “Natural Fertilizers” like fish food, combined with significant biofiltration
All seven are used by planted aquarium hobbyists with varying degrees of success.

If one rate these methods, using a 1 to 10 scale, with 1 being bad and 10 being very good you get this chart:
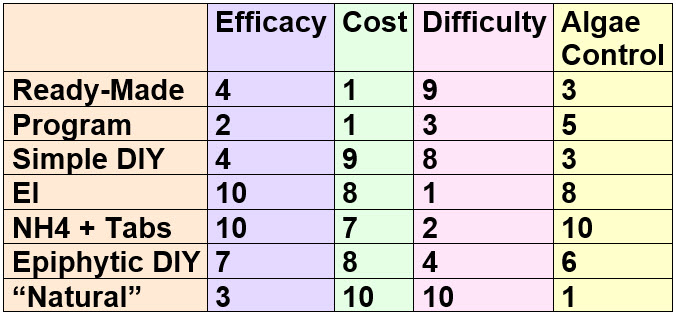
Now everyone will have different properties they want in a fertilizer. So this chart lets one pick and chose.

As is the case in many articles on this website, this article is very long-winded and suitable only for real nerds like the author.
1, Commercial Complete Fertilizers
As far as fertilizer “recommendations” one can go with a good aquarium complete fertilizer and follow the directions on the bottle. The best I could find is the Aquarium Co-op Easy Green fertilizer (and no, I am not an affiliate of Aquarium Co-op). Note ALL commercial liquid fertilizers are expensive and can be duplicated with chemicals from the web at a fraction of the cost.
15.5.1. Ready-Made Fertilizer
2, Commercial “Programs” or “Lines” of Fertilizers
A very expensive method of fertilizing is to go with a commercial fertilization “program” such as the eight-product Seachem line. I’m not exactly a fan of this, to put it mildly. I have a very intense dislike of the Seachem marketing methods, something which probably colors my narrative way too much, but so be it.
To understand the “program” fertilizer methods and products it is best to go to this link:
15.5.2. Fertilizer Programs
Planted Aquarium
3, The Simplest DIY Fertilizer
To make a simple, easy, and cheap DIY nitrate nitrogen fertilizer, simply buy two chemicals then mix per these directions; mix up one liter of hot distilled water with the following:
- 328 grams potassium nitrate (NPK 13-0-44)
- 16 grams mono-potassium di-hydrogen phosphate (NPK 0-52-34)
First, dissolve the dipotassium phosphate into the water and then dissolve the potassium nitrate. This creates roughly one liter of water-based fertilizer solution with a composition of roughly 3-0.6-11 NPK.
More recipes can be found at this link:
15.5.7. DIY Fertilization

4, EI Fertilizing of Planted Aquariums
This DIY method was developed by the excellent scientist Dr. Tom Barr and is extremely effective. Most high-tech planted tank “experts” use this somewhat complex but inexpensive methodology. It is a very time-consuming method of fertilization (weekly water changes) which is why I do not like it. I am lazy. I have attempted to lay out the EI fertilization method in this link:
15.5.3. Estimative Index

5, DIY Ammonium plus Tabs Fertilizer
There is an inexpensive DIY fertilization program that is a little different. It does the following:
- Puts the phosphate and iron into the substrate, not the water column
- Uses ammonium instead of nitrate to supply the nitrogen
The tabs ONLY work with a fine substrate. If one has gravel substrate or an undergravel filter the other fertilizer methods are better. This program can be found at the following link:
15.5.4. NH4 + Tabs Fertilizer
6, DIY Epiphytic Fertilizer
If one has epiphytic plants, this link has a formula for a fertilizer that puts everything into the water column. This fertilizer is the EI fertilization method with ammonium instead of nitrate.
15.5.5. DIY Epiphytic Fertilizer

7. The “Natural” Way: Fish Food as the Fertilizer
Some hobbyists simply use an excess of fish food to fertilize the plants. This can be made to work IF one adds a decent amount of biofiltration while keeping the currents in the aquarium low. This is the link to such an approach:
15.5.6 Fish Food as Fertilizer
Fertilizer Components
Each fertilizer component has its own unique set of characteristics:
- Nitrogen in all its forms is very soluble and is best supplied in the water column.
- Potassium in all its forms is very soluble and is best supplied in the water column. The major use of potassium is to transport various nutrients around vascular plants. As such it is of little use to any form of algae or cyanobacteria.
- Phosphorus (phosphate) gets easily tied up in insoluble calcium, magnesium, and iron compounds. As a result, phosphate becomes relatively “immobile” in a fine substrate and doesn’t readily “leach out” of the substrate. But rooted plants can still access the phosphate in most of the immobile phosphate compounds. Phosphate is thus best supplied in the substrate.
- Iron has two forms. “Ferrous” iron is relatively soluble and plant accessible but easily oxidized to “ferric” iron which is insoluble and not accessible by plants. This oxidation proceeds rapidly in any well-oxygenated environment, especially if the pH is over 7.4. But in a fine substrate, the pH is generally 6.0 to 6.5, oxygen levels are lower, and the iron is readily available to plants. As a result, iron is best supplied through the roots. Iron is only needed in very small but very constant quantities.
- Calcium is sufficient for most plants in all but very soft water, but it is dependent on the plant.
- Magnesium, the forgotten nutrient, is often not sufficient in water for the excellent plant growth of some plants.
- “Micronutrients” are just that, nutrients needed in such tiny quantities that almost any water source provides plenty of them. Only worry about these if one is using RO or distilled water. Note iron is the one micronutrient that IS needed in a fertilizer.

Soft Water
There is one situation where a fertilizer of sorts is called for in all types of fertilization. If the water is very soft (GH less than 3 or dGH less than 72) then it needs both calcium and magnesium additions for most plants. The easiest way to do this is to simply add one level teaspoon of Epsom salts (magnesium sulfate) and one level teaspoon of Plaster of Paris (calcium sulfate and calcium carbonate) to every ten gallons of water change water.
Hard High pH Water
Hard high pH water (GH greater than 10 and pH greater than 7.4) present a problem with iron and phosphate. Under these conditions iron alone is tied up in a matter of hours. Add phosphate to the equation and the iron is tied up in minutes. So with hard high pH water it is very important to add iron and phosphorus to the water column separately at least 24 hours apart, Better yet add both the phosphorus and the iron into the substrate in separate tabs.

Waiting Period
One should wait for one to three months before fertilizing any rooted plants. That gives them a chance to get established and send out nutrient-absorbing roots. Note that it is normal for a new plant to die back when planted in an aquarium. And algae do not die back. Algae begin reproducing as soon as one sets up the planted aquarium. So if one adds fertilizer early, one is just fertilizing algae. One needs to have patience with plants and allow them time to send out roots and get acclimated to their new surroundings. This can take one to three months.
Then gradually ramp up the fertilizer quantities in high-tech aquariums for one to two months. Low-tech aquariums need to be ramped up over six months to a year.
Remember, there are three important things to keep in mind when setting up a planted aquarium:
.
Patience, Patience, and Patience
.

Fertilizer “Ramp-up”
If one has a high-tech aquarium with a lot of light and CO2 injection one might ramp up concentrated high-tech fertilizer amounts such as the EI method or Easy Green by the following progression:
- Week 1 – nothing
- Week 3 – 3% concentration
- Week 5 – 6% concentration
- Week 7 – 12% concentration
- Week 9 – 25% concentration
- Week 11 – 50% concentration
- Week 13 – 100% concentration

If one has a low-tech aquarium with moderate light and no CO2 injection one might ramp up the low-tech fertilizer amounts (typically 20% of the concentration of the high-tech fertilizer) by the following progression:
- Month 1 – nothing
- Month 2 – 3% concentration
- Month 3 – 6% concentration
- Month 4 – 12% concentration
- Month 5 – 25% concentration
- Month 6 – 50% concentration
- Month 7 – 100% concentration
Note the high tech is in weeks while the low tech is in months. This is just a suggestion. I wait longer before adding fertilizer but most hobbyists do not have my patience.

Dosing Pumps
Typically the more times one adds fertilizer to a planted aquarium the fewer algae one will have. So a few freshwater planted tank enthusiasts have taken a page from the reef aquarium hobby and set up what is called “dosing pumps”. They set the pumps up to add fertilizer automatically at exactly the right time every day.
Some enthusiasts use a two-station pump. One pumping nitrogen – phosphorus – potassium, and one pumping iron and micronutrients with the EI method. This is a very good arrangement for a high-tech tank with epiphytic plants.

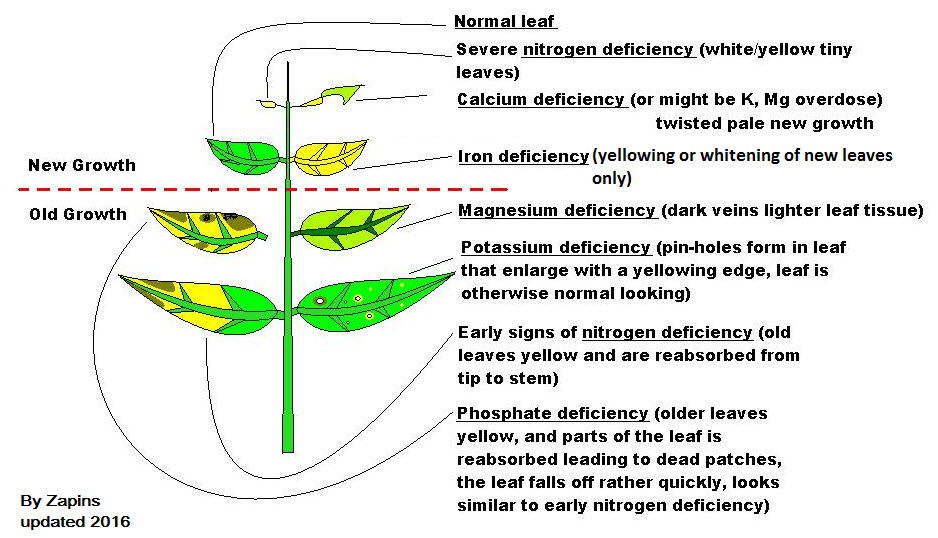
Nutrient Shortage Signs
The following chart from Aquarium Co-op is very useful for fertilizing an aquarium. Just up whatever nutrient is missing for plants:
.
Research Papers
One recent study (“Effects of Substrate Nutrients on Growth of Three Submersed Aquatic Plants”, Gosselin, 2020) of fertilizers was done on three common submerged plants: pondweed, hydrilla, and southern naiad. The Gosselin study by the University of Florida showed ammonium nitrogen gave twice the growth rate of an equal amount of urea nitrogen and six times the growth rate of an equal amount of nitrate nitrogen.
Another study showed foliar uptake of ammonium by Eurasian water milfoil was several times more rapid than uptake of nitrate when both forms of nitrogen were present in the water (Nichols and Keeney 1976).

Note that a whole series of research papers confirm that BOTH algae and vascular plants use ammonium preferentially over nitrate. This is hardly surprising. Let us make it clear:
.
ALGAE IS A PLANT!
.
So playing with nutrient levels to control algae without “controlling” plants with stems and leaves is not productive, other than the use of high levels of potassium for vascular plant growth.

Algae and Fertilization
There are many videos and pieces of “expert advice” that say that “algae” like this or that fertilizer ingredient more than “plants” like this or that fertilizer ingredient. For instance, folks say algae like ammonium while “plants” like nitrate.
But algae like the same thing as “plants” like. Both “higher plants”. also called “vascular plants” (plants that have stems, leaves, and roots) and algae like ammonium better than nitrate. But both can utilize nitrate equally well. So using nitrate will NOT prevent algae. Nor will using ammonium “encourage algae growth” any more than it encourages stem plant growth.

If one only fertilizes in the water column, limiting phosphate will limit algae growth but it will also limit stem plant growth. The only way to use fertilization to give rooted plants a “leg up” on algae is by adding phosphate and iron to the substrate, where algae obviously can’t get to it as algae do not have roots.
But this is not to say that by limiting phosphorus and iron in the water column one can eliminate algae. Algae will grow in VERY nutrient-poor waters. So one cannot COMPLETELY PREVENT algae growth by limiting nutrients. But thriving stem plants prevent algae by putting out chemicals (negative allelopathy). So anything which can give plants a boost will improve the situation.
.

Fertilizers in More Depth
We go into aquarium fertilizers in more depth in the following links:
15.5.1. Ready-Made Fertilizer
15.5.2. Fertilizer Programs
15.5.3. Estimative Index
15.5.4. NH4 + Tabs Fertilizer
15.5.5. DIY Epiphytic Fertilizer
15.5.6 Fish Food as Fertilizer
15.5.7. DIY Fertilization
Planted Aquariums in Depth
The following sections will give you some general guidelines on the easiest ways to get lush plant growth without algae growth in an aquarium:
15. Planted Aquariums
15.1. Planted Aquariums in Depth
15.2. Fish for Planted Tank
15.3. Fish Limitations
15.4. Types of Planted Aquariums
15.6. Carbon Dioxide
15.7. Substrates for Planted Aquariums
15.8. Walstad Aquarium
15.9. High-Tech Planted Aquarium
15.10. Hybrid Planted Aquariums
15.11. Many Fish Many Plants
15.12. Propagating Plants
15.13. Hau Aquariums
15.14. Low Tech Planted Aquariums
2.15. Cycling a Planted Aquarium

Return to Planted Aquarium Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit-making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.


Dave says
In reply to Rod …… If your Malawi tank plants have been doing well without fertilizer, why add fertilizer? The food you are feeding your fish is giving the plants plenty of fertilizer.
Rod Wootten says
Hi
I have just made up the mix that you have recommended and it all seems to have dissolved in to the distilled water ok.
I have a heavily stocked Malawi cichlid tank and I have managed to grow quite a few cryptocorynes, anubia and amazon sword all planted in sand. I also have a plant that i can only remember the name as something cypress. My malawis seem to be happy not to destroy these few plants so i was wondering what sort of dosage I should deliver to the tank weekly?
The tank is 450 ltr but taking the decor in to account I tend to work on 300 litres for any dosage i need to do.
Dave says
In reply to Rahul ….. This is a very complex relationship with living organisms such as fungi playing an important role. Suffice to say the alternatively available application is the best way as becoming unavailable takes time, typically days to weeks.
Rahul says
I have a confusion about iron and phosphate
Since they are known to react with each other, then how come they are supposed to coexist in a planted tank where about 2ppm phosphate is supposed to be there and 0.1 to 0.5 ppm iron ( fe 2+) are supposed to be there?
It seems like only one of them can exist in available form.
And also, adding one, would automatically reduce the availability of the other.
All chelates do is release them slowly, rates depending on pH and chelate complex but it is the releases ions which are absorbed by plants.
But if phosphates are present ( and usually more than 10-20 times iron) the released ions will also form complex and become unavailable.
How to solve the dosing conundrum and explain the presence and availability of both nutrients to plants at the same time?
Sagi says
Hi, let me layout my impressions as a new established planted aquarium maker:
Refrain from fertilization is good for a constraint amount of time as mentioned by you and recommended by other sources online, however this should stop immediately and switched to half to three thirds of suggested daily dosing as soon as any plant start showing signs of yellow or melting leaves. Depriving nutrients from aquatic plants under pretext of feeding algae instead is misleading, even if some algae will consume some of the fertilizer, the most of it will be channeled towards the plants.
The 2hr determining implicitly full daily dosing should start immediately.
I’m handling lots of diatoms, which started without any additions of fertilizers, these are rules of thumb in new set ups.
Sagi says
Suppose I didn’t provide fertilization on new established planted aquarium, how one manages a 3% concentration of fertilization dosing if dosing recommendations are 6 mL (3 pumps) per 50 L water weekly.
Dave says
Fertilizer in the first month doesn’t “boost” plants. It does tend to increase algae growth.
Sagi says
Hi, on the one hand you suggest no Fertilization whatsoever for the first month, and on the other hand you simplify that anything which can give plants a boost will improve the situation.
?
Dave says
In reply to Softie … It shouldn’t add up very fast as the fertilization is per ten gallons of water change water.
Softie says
Hi David, when you were talking about soft water, you mentioned “simply add one level teaspoon of Epsom salts (magnesium sulfate) and one level teaspoon of Plaster of Paris (calcium sulfate and calcium carbonate) to every ten gallons of water change water.”
Will all this add up tremendously over various water changes and top-ups due to evaporative water loss?
I just realized there’s so much information about what i thought was a simple hobby. Reading through your website is like drinking from a fire hose
Dave says
In reply to Ludovic … Every time I made the stuff I had solubility issues. The two ingredient seems to have the least problems with solubility.
Ludovic Lafrance says
For what reason did your recipe for the simple DIY fertilizer (15.5.2) go from three, to two ingredients ?
Dave says
In reply to Anony Mouse …. The problem isn’t with the tubing. The problem is with the fittings on the Jebao. They suck (pun intended) air in because the fittings are not smooth. It is a known problem with the Jebao. Go to a Kamoer X1 Bluetooth pump and the problem will disappear. And use silicone tubing, not the PVC airline tubing.
Anony Mouse says
Love this site! Thanks for sharing your expansive knowledge. I’ve been using Easy Green, but moving towards dosing ferts mixed up from dry ingredients. I have a Jebao dosing pump , I was curious what type of material works best for the airline tubing. The reef community I see lots of folks complaining about different types of airline tubing developing bubbles in the tubing, or leaks. But they are dosing different goop in their tanks than freshwater planted geeks are. So what type of airline tubing do you use for dosing?