
There are two ways to measure CO2 levels in the aquarium:
- Glass reactors (“drop checkers”) are slow, they give you what is in essence an average over three hours or so. And they are difficult to read. But they are dependable under many sets of conditions for those aquarists wanting to hold 30 ppm CO2 or lower. Note Tom Barr (the guru of planted tanks) says the best way to use drop checkers is throw them hard against a wall.
- Using a table and measuring relative pH (the drop in pH from water with no injection to pH of water with injection) is more accurate but somewhat cumbersome to use. I would use this method if one wants over 30 ppm CO2.
This is a somewhat confusing but decidedly boring dissertation only for real nerds like the author.

“Drop Checkers”
The most common method to measure CO2 levels is via “drop checkers”, which work by sitting inside the aquarium water. These are filled with a solution of water and pH reagent known as bromothymol blue.
As the gas in the water reacts with the bromo blue/water solution it changes solution color, ranging from a dark blue, which is a high pH/low CO2, through green to a yellow, which is low pH/high CO2.
The best way to use a drop checker is to fill it with a solution with a dKH of 4° (4 dKH). This 4 dKH reference solution allows monitoring CO2 levels accurately. Using the pH/KH/CO2 table gives that a green color with 4 dKH will give 25 to 35 ppm CO2 which is where many plant enthusiast keep their high tech planted aquariums.

Making up the 4 dKH solution is critical to success of the drop tester. This is how to do it: (Note: You will need a whole gallon of distilled water to do this. It MUST be distilled water)
- Start with 6 cups of distilled water in a clean measuring container
- Add 1/5 LEVEL teaspoon (use the 1/4th measuring spoon and slightly underfill it) Sodium Bicarbonate (baking soda) to the 6 cups of water and mix.
- Let sit for two hours
- Pour out 3 cups of this mix and discard
- Add back 3 cups of distilled water and mix
- Pour out 3 cups of this mix and discard
- Add back 3 cups of distilled water and mix
- Pour out 1 cup of this mix and discard
- Add back 1 cup of distilled water and mix
- Water comes out to a 4 dKH solution
- Wait one day then measure the dKH and adjust accordingly

I personally use the following to get the 4 dKH solution (4 dKH is 99.3 ppm of baking soda)
- Start with the following:
- one gallon distilled water
- one plastic cup labeled #1
- one plastic cup labeled #2
- one measuring cup
- one measuring tablespoon
- one gram scale
- Add 7.3 grams baking soda to cup #1
- Add to one level cup distilled water to cup #1
- Mix and let sit for two hours
- Add one level tablespoon of the mixture in cup #1 to cup #2
- Add to one level cup distilled water to cup #2, mix
- Rinse out cup #1 in distilled water
- Add one level tablespoon of the mixture in cup #2 to cup #1
- Add to one level cup distilled water to cup #1, mix
- Water in cup #1 now comes out to a 4 dKH solution
- Wait one day then measure the dKH and adjust accordingly
This solution will only be good for about one month. Then it has to be remade.

This is what a CO2 drop tester looks like:

Any more blue than that lime green and there’s less than 30 ppm CO2. More yellow means more than 30 ppm CO2. Because the reference 4 dKH solution in the drop checker is separated from the aquarium water via an air gap, the only influence on its pH is via CO2.
Here is a very useful reference:
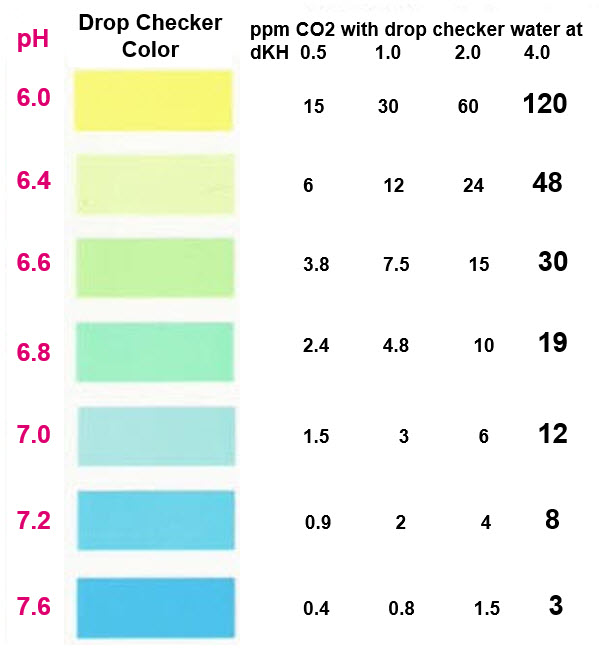
This chart allows one to change the KH so that lower levels of carbon dioxide can be measured. For instance, 2 KH will measure green at 15 ppm CO2 rather than 30 ppm CO2. Simply add only 3.65 grams baking soda to cup #1 to get 15 ppm CO2. To get 10 ppm CO2 measure out 2.43 grams baking soda.
There are disadvantages to this method. The solution inside the drop checker can take several hours to change color, so potentially misleading one into thinking that more gas is required. The larger the air gap between the aquarium water and the drop checker solution, and the more solution volume there is, the more time it will take.
It also requires comparison to a color chart, which can lead to misinterpretation. Be cautious and obtain a darker green rather than a yellow/green color. In very large aquariums it’s not uncommon to use multiple drop checkers to ensure the CO2 is being distributed effectively around the aquarium. Move your drop checker around to determine your gas levels at all parts of your aquarium, especially in high-energy systems that require CO2 levels to be spot on.

CO2/pH/KH Charts
The following chart is can be used to determine the CO2 level in aquariums:
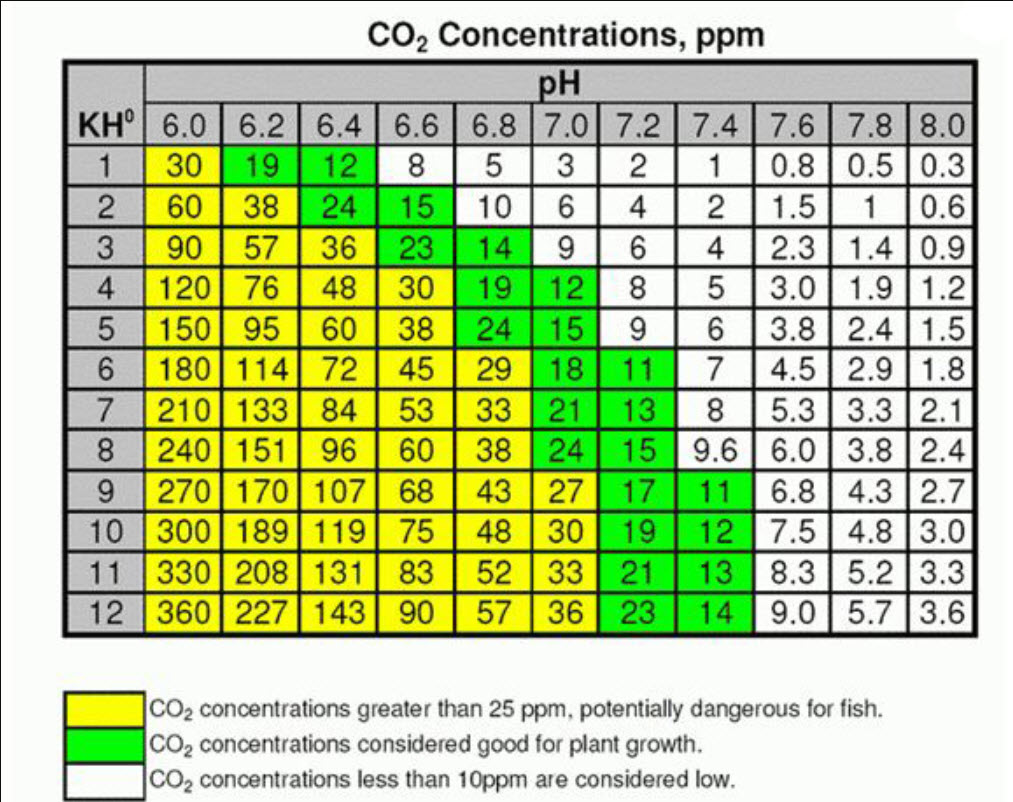
This table relates CO2 content to the pH and KH levels in the water. This relationship is more complex than the table lets on but it is a good starting point. KH (also know as dKH, alkalinity and carbonate hardness) the KH represents the pH buffering capacity of the water. Harder water will have a higher buffering capacity and vice versa. One can find the amount of carbon dioxide in the water by using this table, again, with some reservations.

Using a Table
Paraphrasing Tom Barr: Measure your KH, then see how much you need to reduce the pH to get your target CO2 ppm using any of the many charts on the web. Say you tap water is a KH of 5 and say you want 35-40 ppm of CO2, you should add enough to get the pH to 6.6 and be able to keep it there.
Warning, KH may not be entirely carbonate hardness. This means you will think and believe you have MORE than you do, thus you may be under dosing CO2. These issues will never be the reverse, e.g., you are adding more CO2 than you think. So the error is always on the safe side using this method.
As the KH in your tap drops, say your KH is 1-2 degrees, there’s just not much room for other sources of KH other than carbonate, at 4-5 and above, there may be. So assuming most of the KH is carbonate hardness for a KH or 1 degree is likely okay. Say you want a CO2 of 50 ppm for a KH of 1 degree? the charts typically do not cover those ranges of pH’s, but you can scale using a similar higher KH to see what the pH adjustment would be. So about 5.9 pH would give about 39 ppm and a pH of 5.8 would give about 48 ppm of CO2.
Use a pH meter to read pH accurately. And the Hanna alkalinity (KH) meter is pretty good, same with the Lamotte alkalinity test kit. Test tank water and tap water every month or two as a good measure. Over a season, KH can change from some Tap supplies.

As you depress the pH with CO2 gas, the concentration will increase a lot more (e.g., it’s nonlinear) for each 0.2 units of pH.
Say you have a KH of 3 degrees.
At a pH of 7.0 you would have 9 ppm
At a pH of 6.8 you would have 14.3 ppm
At a pH of 6.6 you would have 22.6 ppm
At a pH of 6.4 you would have 35.8 ppm
At a pH of 6.2 you would have 56.8 ppm
At a pH of 6.0 you would have 90 ppm
Differences between each 0.2 pH units:
5.3 ppm
8.3 ppm
13.2 ppm
21 ppm
33.2

So your pH measurement and observations need to be very good when you use more CO2. If you over do things at the higher ppm’s, it only takes a little bit of change to dramatically increase the CO2. This is one reason why many people fail when adding more CO2 and gas their fish instead. If each 0.2 pH units were only 5 ppm difference, then it would be pretty easy to adjust CO2. This is also a good reason to buy a nice CO2 regulator, needle valve etc.
Since many use the drop checkers and there’s little differences between the colors and those color changes are at best, 0.2 pH, what does this say at the higher ppm’s of CO2? Not much. Or if they use colorimetric pH measure? Similar. A good 0.01 accuracy pH meter is likely the best relative measure for CO2 using pH.

Another factor can throw the measured CO2 accuracy. The CO2/pH/KH relationship assumes that “carbonic acid” from CO2 is the only acidifying compound, but this is not the case for most of us. Nitric acids, sulfates, phosphates, humic acids and other organic matter will all contribute to acidification of aquarium water. This will typically result in pH readings lower than the table assumes, resulting in a false CO2 calculation if one is measuring the KH and pH of the water directly.
If you measure pH/KH when tank is at equilibrium when CO2 injection is off and before the lights have come on in the morning, CO2 levels in the tank will TYPICALLY (but not always) match equilibrium levels with atmosphere, with respect to gas laws – which will be around 2-3 ppm.
Put more clearly, while atmospheric CO2 has a concentration upwards of 400 ppm, the average amount in an open container of pure water stabilizes at around 2-3 ppm, which is called “atmospheric equilibrium”. A tank without CO2 injection will not have elevated levels of CO2, UNLESS there is a lot of rotting organic matter in the aquarium OR UNLESS there are a decent number of fish. Fish and rotting organic matter both put carbon dioxide into the water.

For instance, if I look at the pH and KH of my tank (before CO2 injections starts) and I get a reading of pH of 7 and KH of 6, the table states that I have 18 ppm of CO2. I know that this is PROBABLY a false value caused by other components in the tank that contributed to tank acidity. This is because a tank without CO2 injection will almost always be near atmospheric equilibrium (2-3ppm). This is PROBABLY a false positive. If I take a pH reading later while CO2 is on, I need to factor this in.
But note the term “probably”. If there is some rotting plants or plant roots somewhere in the tank or some sizable fish, they can give up to 10 to 15 ppm of CO2 first thing in the morning. So assuming I have 2 to 3 ppm CO2 CAN be incorrect. And, obviously, a dirted or pelleted tank will have high levels of CO2 first thing in the morning.
In any case, as Tom Barr illudes to above, the added acidity will only drop the true CO2 levels so the acidity numbers are safe.

Electronic pH Shut Off
Some hobbyists use this pH drop to control a CO2 injection system. For instance, if the natural pH is 7.6 in the aquarium and the hobbyist wants to hold a 30 ppm of CO2, then one can set up a pH meter that shuts off the CO2 at a pH of 6.6. The problem with this is that if the pH meter fails or drifts (a very common occurrence), you are simply screwed.
Personally I would use a pH shut-off as a safety valve that shuts off the gas solenoid at a certain pH as a safety feature in addition to using pressure to regulate the flow.

Further Information on CO2 Systems
More information on CO2 in the aquarium can be found at these links:
15.6.3. High Tech CO2 System
15.6.1 Low Tech CO2 Aquarium System
15.6.4. CO2 from Food
15.6.5 Liquid CO2
15.6.2. KH, pH, CO2 Relationships in a Planted Aquarium
4.4.3. Carbon Dioxide and pH
.
Return to Planted Aquarium Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

István says
Dear Dave!
The maths on 4dKH=99.3 ppm baking soda seems off to me.
It’s not just on your site, but also elsewhere (e.g. wikipedia on carbonate hardness: 4dKH=120 ppm sodium bicarbonate), and I just started to question my sanity… 🙂
I think you used 1 dKH= 17.85 ppm [CO3]^2- for the definition of dKH.
Isn’t it defined in CaCO3, just as dGH?
On my end: if 1 dKH= 17.85 ppm CaCO3; Molar weight(CaCO3)=100.1 g/mol; Molar weight(NaHCO3)= 84 g/mol, than:
1 dKH=17.85/100.1*84= 14.98 ppm NaHCO3
Btw, this is my first comment. I discovered your websites a month ago, and this is my favorite read ever since! Thank you very much for it!
Regards,
István
Dave says
In reply to Felipe …. Just add enough to make a strong color. That will vary depending on the source of the bromothymol
Felipe bivort haiek says
Hey Dave thanks again for all the information and explanations you have gathered here.
How much bromothymol blue should we add to the dkh solution?
ben z says
@Dave
You might wish to add that for those who really don’t mind splurging, there is a very good CO2 meter by OxyGuard.
Summerfelt (1996) has an equation for pH ranging between 6.5 to 9.5 that is approximate to 5–10%:
Concentration of CO2 = Alkalinity * 10^(6.3-pH)
units in mg/L. Alkalinity as mg/L of CaCO3.
Bisogni and Timmons (1994) have a very detailed method of measuring CO2 that looks to be very reliable. But it’s complicated with a capital C.
Any thoughts?
Dave says
In reply to Gabor … Yes, you are correct. The chart halfway through this article delineates how to do this change in drop checker KH.
Gabor says
Hi Dave,
Another good explanation; thank you! I guess one could play with the bicarbonate buffer to make the test sensitive in different CO2 concentration ranges. For example, for low-tech, a solution of 0.4 KH could be an indicator for 3 ppm CO2. What do you think?
Anonymous says
Wow! Great explanation and guidance. I’ll likely have much better luck with my planted aquarium now. I use CO2 and monitor it using a drop tester. Things get complicated because I use a liquid fertilizer which requires water changes and our water has a pH of 6.5. So I need to add buffering to get the pH up for my fish. It’s something I’ve only just started doing in part because of some unwanted black algae and also poor plant health. Anyway you’ve given me enough foundation so I can begin to manage things better than I have been. So thank you.
Best regards,
Duncan