
An understanding of the nitrogen cycle is NOT REQUIRED to have a good aquarium. This idea that every hobbyist needs to understand the nitrogen cycle is a myth promulgated by well meaning but ill-informed individuals on social media. But if one is interested in the nitrogen cycle one can read on. This is only for the real nerds, like the author.
This article explains the nitrogen cycle in four layers. The first few paragraphs are the first layer and the simplest explanation. Then the explanation is repeated three times getting more and more complex.

Level 1; Nitrogen Cycle Simplified
Home aquarium hobbyists talk about the “nitrogen cycle” and how everyone must understand the “nitrogen cycle”. This is problematic as many people lack training in chemistry and the “nitrogen cycle” is a chemical process that requires a rudimentary knowledge of chemistry.
The simplest explanation is that fish pee is poisonous to the fish. In an aquarium filter, the fish pee is converted to a non-poisonous compound by colonies of “beneficial bacteria”, which “eat” the fish pee. These “beneficial bacteria” only grow on surfaces with a decent flow of water and they grow very slowly. So one needs lots of water flow through a filter media with a lot of surface area and several weeks to get the nitrogen cycle started, a process called “cycling” in an aquarium. This is the “nitrogen cycle” in a simple nutshell. Easy!
If you don’t cycle an aquarium before adding fish it is possible to damage or even kill the fish (although this happens a lot less than many would lead us to believe).

Level 2; The Nitrogen Cycle in More Depth
A more complete yet accurate explanation of the nitrogen cycle and the whole idea of cycling a tank is surprisingly difficult to come by if one simply Googles it. Google it and you will come up with a bunch of sites that give bad information and which have words in them in blue with underlines on them.
These blue underlined words are links to products that are over-priced and completely ineffective such as “instant start”, “bottled bacteria”, and chemicals. If you click on these links and buy these products both the website and Google receive a commission. Isn’t the profit motive great? These sites are simply not reliable sites to get ANY information on ANYTHING to do with aquariums.
Even the Wikipedia site is completely inaccurate. Remember, the makers of products like bottled bacteria can go up on Wikipedia and change the narrative in their favor. And they do just that.

A more complete explanation of the nitrogen cycle which is not linked to some ineffective aquarium products and is reasonably accurate is from “The Spruce”:
“Stages of the Nitrogen Cycle
There are three stages of the nitrogen cycle, each of which presents different challenges.
Initial stage: The cycle begins when fish are introduced to the aquarium. Their feces, urine, as well as any uneaten food, are quickly broken down into ammonia (NH3). Ammonia is toxic to fish at levels over 2 ppm. Ammonia usually begins rising by the third day after introducing fish.
Second stage: During this stage Nitrosomonas bacteria oxidize the ammonia, thus eliminating it. However, the by-product of ammonia oxidation is nitrite, which is also highly toxic to fish. Nitrites levels as low as 1 ppm can be lethal to some fish. Nitrite usually begins rising by the end of the first week after introducing fish.
Third stage: In the last stage of the cycle, Nitrobacter bacteria convert the nitrites into nitrates.
Nitrates are not highly toxic to fish at low to moderate levels. Routine partial water changes will keep the nitrate levels within the safe range. Established tanks should be tested for nitrates every few months to ensure that levels are not becoming extremely high.”
This is a reasonably accurate explanation of the nitrogen cycle. It took several Google pages to find it among all the fake marketing hype stuff. It does overstate the toxicity of ammonia but that is a minor error.

Myth: There is a Fourth “Denitrifying” Step to the Nitrogen Cycle
One huge myth about the nitrogen cycle, promulgated by the manufacturers of some “denitrifying media” such as BioHome and SeaChem De*Nitrate, is that there is a fourth step in the nitrogen cycle in the aquarium, namely the reduction of nitrates to nitrogen gas. Doesn’t happen and simply can’t possibly happen in any aquarium. We debunk that myth in the link below:
7.5. Denitrifying Media
.

Level 3; The Nitrogen Cycle in Charts and Graphs
There are several general types of bacteria and archaea involved in oxidizing somewhat toxic ammonia (NH3) to relatively safe nitrate (NO3) in the freshwater aquarium. These are called “nitrifying” bacteria and archaea. The difference between bacteria and archaea is unimportant and we will ignore it in all these discussions. We will use the term “beneficial bacteria” for both the bacteria and the archaea.
These “beneficial bacteria” are responsible for what is known as the “nitrogen cycle” in an aquarium. This cycle can be shown graphically as:
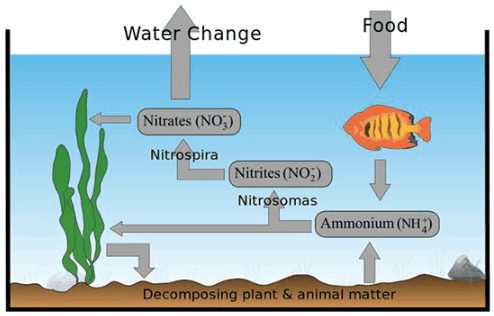
“Nitrosomas” and “Nitrospira” are bacteria. In the hobby, these bacteria are called “beneficial bacteria”. These “beneficial bacteria” do this oxidation of ammonia in two steps. The first step is to oxidize ammonia (NH4) to nitrite (NO2). Nitrite (NO2) is then oxidized to nitrate (NO3), which is much less toxic than either ammonia or nitrite. The cycling process for a typical aquarium can be illustrated graphically as follows:
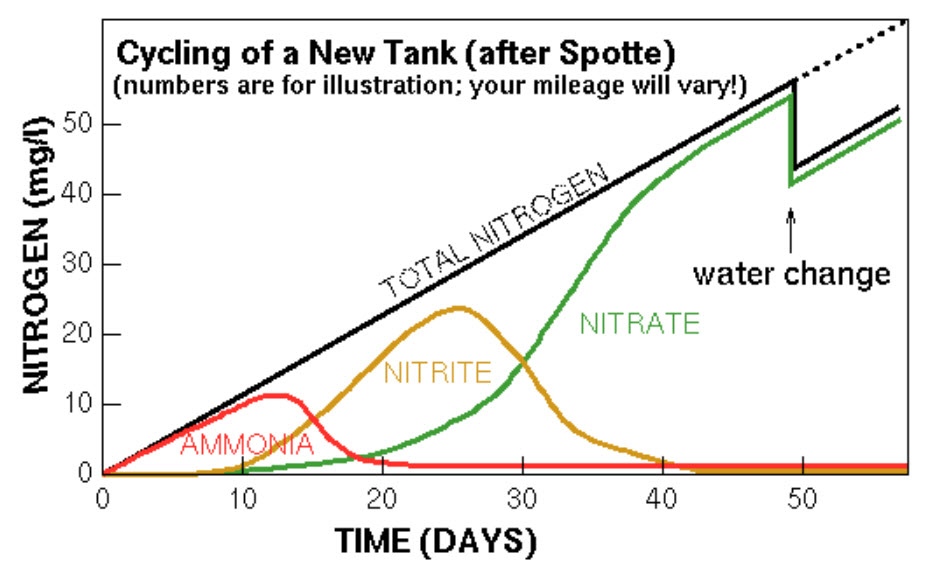
First off, the ammonia rises. Then typically but not always the nitrite rises and falls. After about four to eight weeks the levels of nitrite and ammonia become less than 0.25 or zero, nitrate typically but not always, goes up to over 10 ppm and the cycling is complete. Note the API ammonia test can give 0.25 ammonia with ultrapure distilled water, so 0.25 or less is the goal for ammonia.
This graph is idealized. It sometimes is not what happens during cycling. The levels of ammonia and nitrite can go up and down quite randomly during cycling and produce a graph with much more “noise”. Sometimes nitrite never shows up. And sometimes nitrate never shows up. The nitrogen cycle is a natural process. And Mother Nature is just not very predictable.
All the “beneficial bacteria” involved in this ammonia oxidation need amounts of oxygen (greater than 80% water saturation), carbon dioxide, and ammonia to thrive. They also all need a surface to hold on to, none can reproduce in a free-swimming mode. They are all very slow to multiply, with NO exceptions.

Level 4; The Exact Science in Big Complex Terms
The exact science of nitrifying bacteria is not essential to understanding biofiltration. The science is also very confusing, with a lot of big words to which even the experts give conflicting definitions. But some like to get a very in-depth analysis of it.
Any discussion of nitrification must use a university study published in a reputable journal. There are a huge number of “fake science” papers out there written by the suppliers of aquarium products. The most in-depth and accurate assessment we could find was in the excellent scientific paper by Anne Bernhard (Department of Biology, Connecticut College), “The Nitrogen Cycle: Processes, Players, and Human Impact”, Nature Education Knowledge 3(10):25, 2010:
“Nitrification is the process that converts ammonia to nitrite and then to nitrate and is another important step in the global nitrogen cycle. Most nitrification occurs aerobically and is carried out exclusively by prokaryotes. There are two distinct steps of nitrification that are carried out by distinct types of microorganisms.
The first step is the oxidation of ammonia to nitrite, which is carried out by microbes known as ammonia-oxidizers. Aerobic ammonia oxidizers convert ammonia to nitrite via the intermediate hydroxylamine, a process that requires two different enzymes, ammonia monooxygenase and hydroxylamine oxidoreductase. The process generates a very small amount of energy relative to many other types of metabolism; as a result, nitrosofiers are notoriously very slow growers. Additionally, aerobic ammonia oxidizers are also autotrophs, fixing carbon dioxide to produce organic carbon, much like photosynthetic organisms, but using ammonia as the energy source instead of light.
Unlike nitrogen fixation which is carried out by many different kinds of microbes, ammonia oxidation is less broadly distributed among prokaryotes. Until recently, it was thought that all ammonia oxidation was carried out by only a few types of bacteria in the genera Nitrosomonas, Nitrosospira, and Nitrosococcus. However, in 2005 an archaeon was discovered that could also oxidize ammonia (Koenneke et al. 2005).
Since their discovery, ammonia-oxidizing Archaea have often been found to outnumber the ammonia-oxidizing Bacteria in many habitats. In the past several years, ammonia-oxidizing Archaea have been found to be abundant in oceans, soils, and salt marshes, suggesting an important role in the nitrogen cycle for these newly-discovered organisms. Currently, only one ammonia-oxidizing archaeon has been grown in pure culture, Nitrosopumilus maritimus, so our understanding of their physiological diversity is limited.
The second step in nitrification is the oxidation of nitrite (NO2-) to nitrate (NO3-). This step is carried out by a completely separate group of prokaryotes, known as nitrite-oxidizing Bacteria. Some of the genera involved in nitrite oxidation include Nitrospira, Nitrobacter, Nitrococcus, and Nitrospina. Similar to ammonia oxidizers, the energy generated from the oxidation of nitrite to nitrate is very small, and thus growth yields are very low. In fact, ammonia- and nitrite-oxidizers must oxidize many molecules of ammonia or nitrite in order to fix a single molecule of CO2. For complete nitrification, both ammonia oxidation and nitrite oxidation must occur.
Ammonia-oxidizers and nitrite-oxidizers are ubiquitous in aerobic environments. They have been extensively studied in natural environments such as soils, estuaries, lakes, and open-ocean environments. However, ammonia- and nitrite-oxidizers also play a very important role in wastewater treatment facilities by removing potentially harmful levels of ammonium that could lead to the pollution of the receiving waters. Much research has focused on how to maintain stable populations of these important microbes in wastewater treatment plants. Additionally, ammonia- and nitrite-oxidizers help to maintain healthy aquaria by facilitating the removal of potentially toxic ammonium excreted in fish urine.”
How’s that for complicated?

Acidification
Many hobbyists note a significant drop in pH, like 7.4 to 6.0, during cycling. The end product of the cycling process is “nitrate” NO3, per conventional wisdom. And, again per conventional wisdom, “nitrate is acidic”. Technically that is incorrect. Nitrate is not the end product of the nitrogen cycle and nitrate is not acidic. Nitric acid and hydrogen ions are the end product of the nitrogen cycle and both are acidic.
What people fail to understand is that “hydrogen nitrate”, or “nitric acid”, is what forms in pure distilled water as the final product in the nitrogen cycle. In other words, “nitrate”, NO3, is actually “nitric acid” HNO3 in pure distilled water.

Let us look at the decomposition products of a typical simple amino acid; Tyrosine C9H11NO3. When a fish eats the tyrosine in a protein in their food, they use oxygen to decompose it.
C9H11NO3 => 9CO2 + 5H2O + NH3 .
The CO2 gases off, leaving water and ammonia. The ammonia is released to the aquarium water where bacteria use oxygen to oxidize the ammonia to nitric acid (HNO3) and hydrogen ions:
2NH3 + 3O2 => 6H+ + 2NO3– => 4H+ + 2HNO3
The 4 hydrogen ions and the nitric acid are acidic and acidify the water if there is not carbonate buffering to absorb them.

In zero KH water you might see a pH drop from say 7.4 to 6.0 pH. At 6.0 pH the bacteria stop working on the ammonia and there is no further acidification. But note that the difference between 7.4 and 6.0 is a VERY small amount of nitric acid. And you must have a low pH, and low KH water to see the drop.
I have “hard” well water with lots of calcium carbonate in it (i.e. a high GH and KH) and a pH of 8.2. I don’t see any drop from 8.2 when doing “normal” cycling because I form nitrate, not nitric acid. The hydrogen ions and nitric acid of cycling in my high KH, calcium carbonate-filled well water releases the carbon dioxide from the calcium carbonate and forms calcium nitrate instead of nitric acid. The pH is buffered by the KH and doesn’t drop.
2HNO3 + CaCO3 => Ca(NO3)2 + H2CO3 => Ca(NO3)2 + H2O + CO2
But when I did some of my long experiments (for instance on deep sand beds), my pH dropped WAY down. I depleted all the calcium carbonate and formed hydrogen ions and nitric acid.

A Minor Semantics problem
In the hobby everyone emphasizes how important it is to “cycle” an aquarium and filter. This use of the term “cycle” is technically a misnomer, an incorrect use of the word. The verb “cycle” is defined in biology as:
“a recurring series of events or metabolic processes in the lifetime of a plant or animal.”
Since you only “cycle” an aquarium once, the usage is incorrect. But this is a minor issue. It is common usage among the hobby to use the term “cycle” to mean:
“the establishment of a stable nitrogen cycle in an aquarium filter”
Since the nitrogen cycle is actually a repeating cycle this doesn’t stretch the meaning of the word too much.

Further Data
The actual science of “beneficial bacteria” and biofiltration is very complex and whole books can be written on it. This science is reviewed in the following links:
2.11. Inoculate for Cycling
2.12. Beneficial Bacteria
2.14. The “Mature” Aquarium
The topic of filtration and cycling are closely related. So if one is interested in delving deep into the science and the calculations behind all aspects of filtration the following is pertinent:
6.2. Biofiltration
6.2.1. Detritus Explained
6.2.2. Brown Gunk
6.2.3. Cloudy Water
.
Return to Cycling Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit-making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Susan says
I loved this, thank you.