
Carbon dioxide is rarely a factor in most fish only aquariums. Where it is slightly important in a fish only aquarium is in the consideration of pH. The various relationships of carbon dioxide in the aquarium is a very convoluted chemical dissertation best left to the obsessive compulsive among us. Read on only if you are a real nerd (like the author).
Carbon Dioxide Relationships in the Aquarium in Depth
Carbon dioxide makes up only 0.04% of the gases in the atmosphere. When the water in a aquarium is aerated this small amount of carbon dioxide dissolves into the water as the gas CO2. It is a firmly held belief in the aquarium hobby that this CO2 combines with water to form “H2CO3” or “carbonic acid”.

Note that, in truth, H2CO3, or “carbonic acid”, is not a “real” compound. Many make the error of assuming this “carbonic acid’ is a weak acid, and does not ionize to a great extent, remaining largely H2CO3. The truth of the matter is that there are no H2CO3 molecules in aqueous solution. They don’t exist. What we call “carbonic acid” is CO2 dissolved in pure water in equilibrium with slight amounts of H+ and HCO3–.
CO2 + H2O <==> H+ + HCO3– …….. Ka is small
A small Ka means that the equilibrium lies far to the left, and that almost all the CO2 stays CO2 and certainly doesn’t form molecular H2CO3. If the water in which the CO2 is dissolved is NOT distilled and has a somewhat high pH (say 7.5) then the excess OH– will combine with the CO2 to form HCO3– salt. Above 8.0 pH the excess OH– will combine with the HCO3– to form H2O and CO3-2 Salts. These reactions are what makes the carbonate and bicarbonate salts “buffers”.

This complicated relationship is shown by what is known as a Bjerrum plot:
.
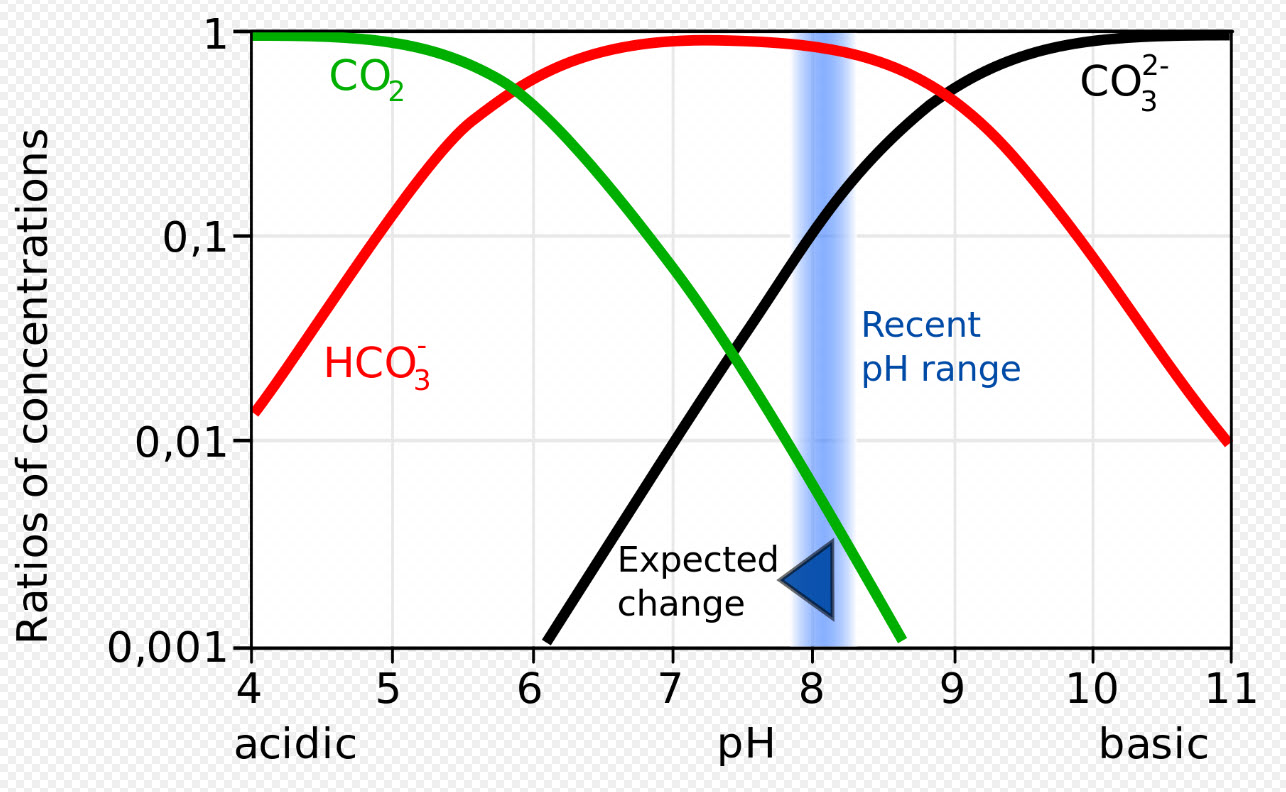
Note that the scale on the left side is logarithmic.
When CO2 dissolves in water the small amount of H+ formed does drop the pH but it doesn’t do it very much (newsflash: because pH is logarithmic around 7.0 a pH drop from 7.5 to 6.5 is a VERY, VERY small difference in amount of H+). And if there is a high pH like 8.5 from carbonate ions then the carbonate ions will “buffer” (i.e. remove) the H+ ions of the CO2 acidification by forming water and bicarbonate.
As a result, ONLY carbon dioxide dissolved in pure water does NOT buffer in any way. Only a carbonate salt or bicarbonate salt will buffer. These salts include calcium carbonate (limestone) and sodium bicarbonate (baking soda).

This association goes back and forth constantly with fish respiration, photosynthesis, and aeration. This can move the pH of the water from 6.0 to 8.5 and back again in hours. In a pond in nature, at 2 PM on a sunny day, the water can be 8.8 pH. By 6 AM the water can be 6.5 pH. (Wurts and Durborow, University of Kentucky, 1992, Masser, Texas A&M University, 2012). Planted aquariums or even aquariums with a lot of algae in them can have the same pH swings.
Pure water (so-called triple distilled water) with no gas dissolved in it has a pH of 7.0. Leave this pure water open to the atmosphere for three days and the pH can go down to 6.8 because of the absorption of amounts of carbon dioxide from the atmosphere. Carbon dioxide in pure water will stabilize at about 2-3 ppm of carbon dioxide.

If ones tap water has little carbon dioxide in it it can come out of the tap a say 7.8 pH. Then as it absorbs atmospheric it can drop to 6.8 pH after a few days. Conversely if the tap water is high in carbon dioxide it can come out of the tap at 6.2 and then stabilize in the aquarium at 7.8 pH after a few days.
To give one some idea of the preponderance of these chemical species, oxygen is 21% of the atmosphere. Carbon dioxide is 410 ppm in the atmosphere or 0.041%. At 0.04% the concentration of carbon dioxide is 525 times less than oxygen in the atmosphere. But carbon dioxide species take up 2-3 ppm in pure water while oxygen in pure water will only be 8 ppm.

What allows CO2 to be dissolved in water so much better than O2 is because, on the CO2 molecule, there is a slight negative charge on both the oxygen atoms and a slight positive charge to the carbon atom. This gives CO2 something called a “quadrupole moment”, meaning the slightly positive hydrogen atom of water is slightly attracted to the two oxygen atoms of the CO2 and the slightly negative atom of the oxygen in the water is very slightly attracted to two sides of the carbon atom of the CO2.
So each CO2 molecule is surrounded by four water molecules in a very weak association. Oxygen forms no such association and thus doesn’t dissolve as well as carbon dioxide.
Note that carbon dioxide, carbonate hardness (KH) and pH are interrelated in very convoluted and complex chemical pathways. This is the “Bermuda Triangle” of the aquarium chemistry. Go there and you may never come out. I’m a chemist and I don’t try to control this triangle. Just keep the pH between 6.5 and 8.5 and total dissolved solids above 60 and ignore the rest.

Carbon Dioxide in More Depth
For more on the Carbonate relationships with carbon dioxide and pH one can go to this article:
4.5.2. Carbonate Hardness
Planted Aquarium
If one wants to explore the Bermuda Triangle of Fishkeeping in a planted aquarium one can go to these links:
15.6 Carbon Dioxide in a Planted Aquarium
15.6.2. KH, pH, CO2 Relationships in a Planted Aquarium
pH in Depth
For those interested in a more in depth discussion of pH click on the following links:
Link to general discussion of pH:
4.4. Aquarium pH
Link to a more in depth discussion of pH and how unimportant it is in the aquarium:
4.4.1. pH is not Important

A difficult to understand part of pH is the concept of “buffering”. Some consider it important to buffer an aquarium.
4.4.2. Buffering the Water
There are situations when one has a pH greater than 8.5 and one needs to drop the pH. This link covers how to safely drop the pH of water
4.4.4. Dropping pH
And sometimes water out of the faucet that has set for two days has a pH less than 6.5 or 7.0 and needs to be raised. This link covers how to safely raise the pH:
4.4.5. Raising pH

Many people think that fish which have been bred in a wide range of waters can thus tolerate a wide range of water. This is simply a myth.
4.7. Fish Tolerance to pH
And many think that fish must be kept in a very stable pH or temperature and that rapid changes are detrimental to the fish. This is yet another myth.
4.8. Stability Isn’t Important
.
Return to Temperature, pH, KH and GH
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Gabor says
Well, that explains it, I was missing this information, thanks a lot!
Dave says
In reply to Gabor ….. Henry’s law only applies to non-polar gases such as O2 and N2. Carbon dioxide is quadripolar and held in a loose polar relationship with four water molecules.
Gabor says
Hi Dave,
Lots of very useful things here; I always find something good to read, thanks! My only slight concern is that I find it to be a bit difficult to navigate the site to return to something I have visited before. I am not sure if I asked this yesterday or not (if I pushed the “Post Comment” button after writing the comment); and I can’t remember on what page I was exactly, so I’ll post it again (sorry if this is a duplicate, ignore it then, I will bookmark this one):
You say that in equilibrium with atmospheric air, the CO2 concentration should be 2-3 ppm in water (I find similar values elsewhere as well). Still, when I try to apply Henry’s law to calculate the CO2 concentration, I get about 0.6 mg/l:
1.449 g/l (CO2 solubility at 1 atm, 25 °C) * 0.0004 (CO2 fraction in the air, 0.04%, CO2 partial pressure is proportional to that) = 0.5796 mg/l (or ppm)
What am I missing here?