
Aquarium water pH is simply not very important. MYTHBUSTER:
.
Most fish have a large tolerance range when it comes to pH. And all fish accept very wide and sudden changes in water pH quite readily.
.
The Simple Explanation of pH
Most folks make pH very complicated, with terms like “logarithmic” and “hydrogen ion”. pH is simply the acidity of the water. The lower the pH the more acid everything is. pH is centered around 7.0. Above 7.0 is alkaline (there are more OH– ions than there are H+ ions) and below 7.0 is acid (there are more H+ ions than there are OH– ions).
An illustration from something everyone experiences is helpful.
Human stomachs are normally at pH levels of around 2 to 3. This is moderate acid. When we eat food, our stomach produces acid to help digest the food. However, when we eat the wrong foods, our stomach produces too much acid and pH levels may drop to 1, which is very acid. A pH of 1.0 is ten times more acid than a pH of 2.0. This 1.0 is when we start to experience pain, heartburn and “acid stomach”. Then we take antacids to help.
An antacid typically will have a pH of roughly 9. This is called an alkaline substance, it is the opposite of an acid substance. So, when we take the antacid the acid pH in our stomach goes from 1 to very roughly 4 to 7 (depends on how many antacid tablets we take) and the heartburn and acid stomach go away. Combine a low pH acid component with a high pH alkaline component and the two “neutralize” each other.

The Topic of pH in More Depth:
pH is what is termed a “logarithmic” relationship. The nature of “logarithmic” is that 6.0 pH is only slightly acid, 5.0 pH is 10 times more acid than 6.0, 4.0 pH is 100 times more acidic than 6.0 pH and 3.0 pH is 1,000 times more acidic than 6.0 pH. So:
- 13 pH very alkaline, like lye
- 9-12 pH alkaline
- 8 pH very slightly alkaline
- 7 pH neutral
- 6 pH very slightly acid
- 2-5 pH acid
- 1 pH very acid, like muriatic pool acid

pH and the Fish
Pure water has a pH of 7. Fish like to live in water which is close to this pH. Some fish come from native waters which have water a little acid and some fish come from native waters that are a little alkaline.
The following are the ranges for tropical fish in the artificial environment which is an aquarium:
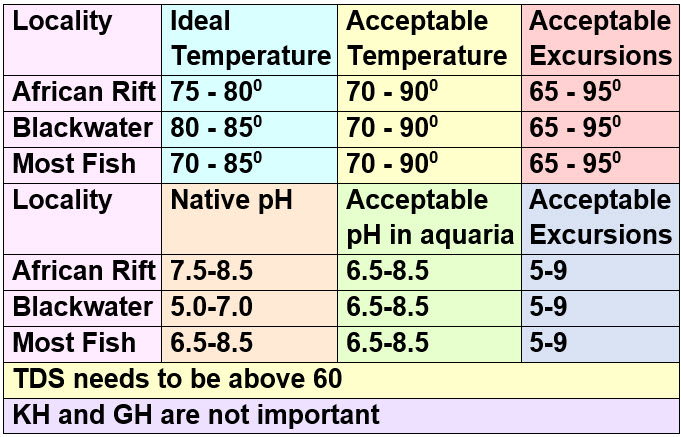
Aquarium pH’s above 8.5 are not recommended because a chemical found in the aquarium produced by fish called ammonia becomes very toxic and because the ammonia oxidation process (“cycling”) slows down. Aquariums pH’s below 6.0 are not recommended because a chemical found in the aquarium called nitrite becomes very toxic and because the ammonia oxidation process (“cycling” or the “nitrogen cycle”) stops, at least for the short term.
Now a heavily planted tank with only a few small fish in it can go acidic will little effect on the fish simply because the ammonia and the nitrite production is very low. So it is possible to have a very nice planted tank with acidity in the 5.0 to 6.0 range. Just do not try it with a lot of fish.
Note that there is a type of organism called an “archaea”, which is sort of a primitive bacteria, which can do oxidation of ammonia at very low pH. But archaea are extremely slow growers which can take four to twelve months to “cycle” an aquarium with an acid pH.

It is common to put fish in aquariums with a pH outside their native ranges. This is because pH is just not important. The author has raised and bred Lake Malawi cichlids, Lake Tanganyikan cichlids, guppies, mollies and platies in the soft acid water of Connecticut. The author has also raised and bred blackwater rams in the hard alkaline waters of Florida.
Oscars, a fish native to the very soft, acid waters of the Rio Negro river (there is some debate about this), have naturalized in the hard alkaline waters of Florida and are an invasive species there.
That being said, if one is breeding fish, it is a good idea to have the fish in a pH similar to their native waters. There are some fish which only breed well in their native water pH. This seems to be especially true of blackwater fish.

The hobbyist should not “chase” pH with chemicals. It is not dangerous, contrary to popular myth. But chasing pH will just frustrate the hobbyist. The pH in an aquarium changes all the time quite unpredictably. A swing as large as 6.5 pH to 8.0 pH in ten hours is quite common in aquariums. Also the test kits for pH are simply not very accurate. Indeed the electronic meters for testing pH are sometimes more inaccurate that the test kits as they need careful storage and calibration.
All aquariums should have a bag of crushed coral or dolomite in their filter. This buffers the water to a pH of 7.6 to 7.9. This pH is a great pH for all fish. And it simply removes all concern about a tank “crashing” and going to a very low pH, with ammonia oxidation stopping. But note that the “7.6 to 7.9” is an average. At times an aquarium with crushed coral can be 6.9 pH and at times it can be 8.5 pH. This is because of variations in dissolved carbon dioxide which occur naturally in any aquarium.

pH in Depth
For those interested in a more in depth discussion of pH click on the following links:
4.4.1. pH is not Important
A difficult to understand part of pH is the concept of “buffering”. Some consider it important to buffer an aquarium.
4.4.2. Buffering the Water
pH goes up and down constantly in an aquarium because of carbon dioxide and how it interacts with water. This is another relatively complex topic:
4.4.3. Carbon Dioxide and pH

There are situations when one has a pH greater than 8.5 and one needs to drop the pH. This link covers how to safely drop the pH of water
4.4.4. Dropping pH
And sometimes water out of the faucet that has set for two days has a pH less than 6.5 or 7.0 and needs to be raised. This link covers how to safely raise the pH:
4.4.5. Raising pH
Many people think that fish which have been bred in a wide range of waters can thus tolerate a wide range of water. This is simply a myth.
4.7. Fish Tolerance to pH
And many think that fish must be kept in a very stable pH or temperature and that rapid changes are detrimental to the fish. This is yet another myth.
4.8. Stability Isn’t Important

.
Return to Temperature, pH, KH and GH
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Dave says
In reply to Matt L …. A Hau bottle will drop the pH half a point or so. But plants and light will use the carbon dioxide and the pH can go up.
And just add a handful of crushed coral in a bag in the filter. There is no formula.
Matt L says
Hi Dave,
You mentioned that adding crushed coral to the filter is recommended in any tank, does this apply to a heavily planted tank with CO2 (Hau Bottle) as well? Will the PH buffer to the same level as well?
How much crushed coral should be added? My current filter for the 20 gallon is a bottom to top HOB (Tidal 55) with 100% 30PPI form, what is the calculation if any for the amount of crushed coral.