
General hardness, GH, in the water basically reflect the amount of the elements Calcium and Magnesium one has in the water. Calcium and Magnesium is what forms scale and crusts in your toilet and bathtub tiles (and your aquarium). Calcium and Magnesium are elements which don’t dissolve very well in water.
So, when given the chance they come out of solution and form a crust where it isn’t wanted. If you have very hard water your pipes can become plugged and stop flowing due to these deposits. GH is simply a measure of this hardness.

GH is not important for adult fish. Adult fish have mechanisms which are quite efficient at coping with large variations in GH. But fry are less able to deal with these variations. So if one is raising eggs, larvae and fry one in some cases one should follow these guidelines:
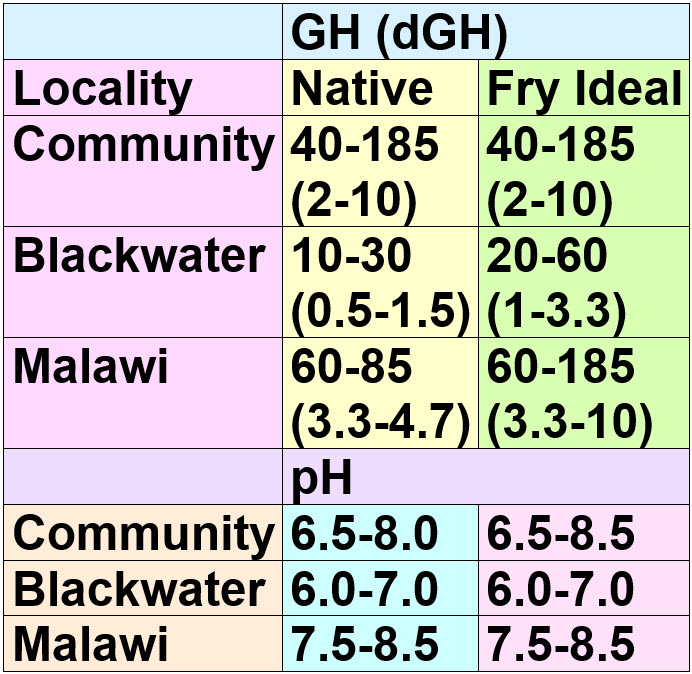
Note that livebearers follow the community guidelines. Livebearers are NOT brackish water or even hard water fish. They are native to rivers and streams with water of moderate hardness and salt content.
Also note that many think Lake Malawi is very hard. It isn’t. The Malawi data in the chart above is from the seminal work of Talling in 1965 on Lake Malawi Chemistry, updated by Joe Gargas, former Director of Research and Development for the Wardley Corporation and an expert in water chemistry and treatment.
Also note that individual types of fish vary greatly in what they need for spawning and fry viability. Some fish such as cardinals and clown loaches have never spawned in captivity to my knowledge. Others such as neons and discus need perfect conditions for spawning. The Central American and African cichlids spawn so well under all water conditions they are just plain a nuisance (try giving away convicts sometime!). Livebearers also breed under virtually all water conditions.

As in many articles on this site we now go into GH in more scientific terms. This repetitious series of explanations is only for the real nerds, like the author.
General Hardness in Greater Depth
“General hardness” (GH) measures the cations (+ charge) for calcium and magnesium as ppm of calcium carbonate (CaCO3) equivalents.
There are many terms used interchangeably and often incorrectly for GH. Here is a chart:
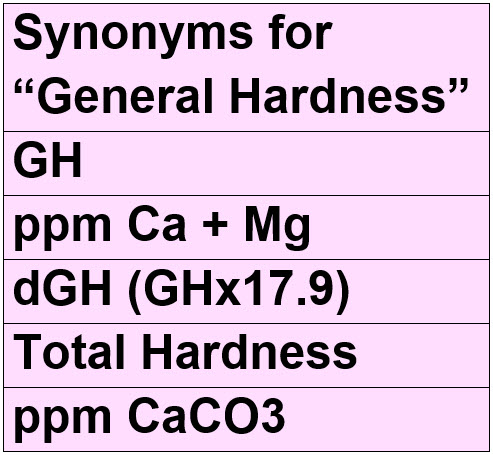
It would make sense to measure the general hardness in # of ions/liter or molarity, but this is not used except in scientific articles. The common unit found in the literature is degrees of general hardness dGH from the German system (dGH or “degree German Hardness”, in German “deutsche Härte”).
Converting from dGH to GH can be accomplished by multiplying by 17.9. Conversely converting from GH to dGH can be accomplished by dividing by 17.9. One dGH is equal to 17.9 GH, 17.9 mg/L or 17.9 ppm. Waters that have moderate to high levels (50 mg/L or greater) of GH usually have a basic pH but not always. If the hardness is caused by sulfates such as calcium sulfate or magnesium sulfate (Epsom salts) the pH can be quite low.
To complicate matters further there is a unit of general hardness called the “grain”. This number is only slightly less than the dGH number. There is also “parts per million”, “millimoles”, “French hardness” and “Clark hardness”. If you are in a country which uses these terms just Google it.

General Hardness and Fish Health in Depth
General hardness from 20 GH (1dGH) to 300 GH (17 dGH) is just fine for all adult fish. I’ve kept rams native to the very low GH blackwaters of the Orinoco in water at 180 GH (10 dGH) in Florida. And I’ve kept mollies in very soft acid water in Connecticut. It is far more important to have crystal clear, bacteria free water than to have the proper GH hardness.
Oscars, a fish native to the very soft, acid waters of the Rio Negro river (note some dispute this), have naturalized in the hard alkaline high GH waters of Florida and are an invasive species there. Guppies have naturalized in water around the world of all different hardness’s.
A paper was done on a livebearer closely related to mollies, guppies and platies, Poecilia velifera (“The effects of water type on growth, survival and condition of Poecilia velifera”, Semra Küçük, 2010). This compared the growth rate of this livebearer in hard water (250 GH, 340 KH) versus soft water (18 GH, 72 KH). There was no difference in growth rate or mortality in the two groups.
Some general hardness is needed for healthy snails and shrimp. See the article on shrimp for more on this.
17.10. Shrimp

There are many tropical fish farms in Florida on wells. These fish farms all have very high GH water as they have limestone underneath them. And they raise all sorts of fish from tetras from the Amazon to danios from South East Asia to cichlids. Most of the fish do just fine in ponds or tubs on well water. Only a few fish need RO water in order to breed.
The science is rather firm that GH does not affect adult fish very much at all. Adult fish have various coping mechanisms for hardness which adapt quite well to greatly varying hardness. Fish eggs, larvae, and fry are less able to cope. But even then the acceptable ranges found by researchers are quite broad. This is a compendium of the research:
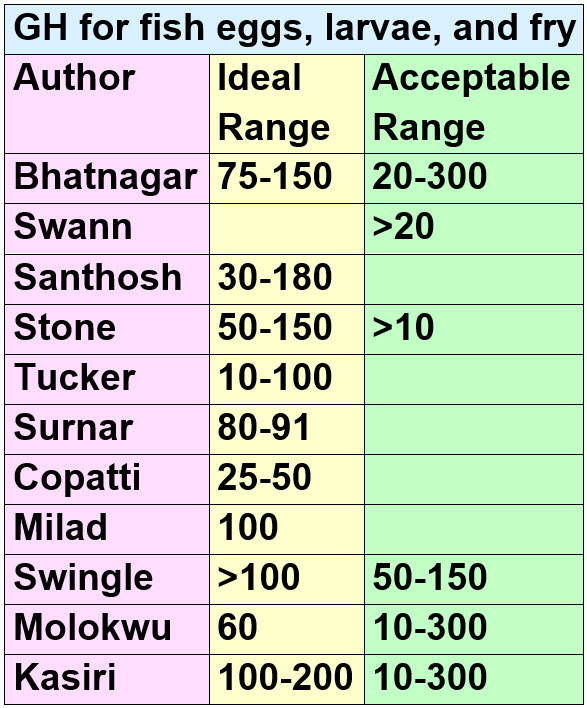
The “acceptable range” numbers are quite broad, typically 20-300 GH. This is because GH is just not that important for fish. Five of these research studies had this broad range.
Each of these studies looked at a different fish species larvae and fry so the variation is no surprise. Also note that fish larvae and fry need to either get calcium for growth of their bones from the water they swim in or from the food they eat. If fry are fed a diet deficient in calcium they die in very low GH water. This is a confounding factor in the above studies. Another confounding factor is that high GH generally gives high pH. And some fish fry (i.e. angelfish fry) don’t do well in high pH even if the water has a low GH. Also note that none of these studies looked at rift valley cichlids.

There is one paper which has come up occasionally on social media: “Water Hardness Influenced Reproductive Dynamics in Two Freshwater Fish Species; Poecilia reticulata and Betta splendens”, Krishnakumar, 2020. This paper claims guppy fry survive best in water modified by the addition of pure calcium carbonate powder to 900 ppm (900 GH), i.e. extremely high. The paper than goes on to site two other studies which the paper claims support the very high GH level.
There are some problems with this study. First off it is impossible to obtain 900 ppm (900 GH) of calcium carbonate in water using purified calcium carbonate. With pure calcium carbonate the highest GH that can be achieved is about 350 ppm. Secondly the two papers cited did not even test high levels of hardness. If one looks up the papers, the water used in one had 60 ppm (60 GH) and the other had 100 ppm (100 GH). These are NOT high levels of hardness. Thirdly the lowest level in the study, 150 ppm (150 GH), is identified by the author as “soft water”, which it isn’t.

And this study is in conflict with no less than eleven other studies in the chart above. This appears to be a translation problem where the numbers are off by a factor of ten. If one assumes the numbers are off by a factor of ten, this study says the acceptable is greater than 15 ppm (15 GH) and the ideal is 54 to 90 ppm (54 to 90 GH), which puts this study right in line with the other studies. It also makes the references valid and 15 ppm (15 GH) is indeed “soft”.
This paper is from Sri Lanka where the native language is probably the Sinhala language. Somehow the units translation was incorrect.
Carbonate Hardness.
To examine the other type of hardness, namely carbonate hardness or KH, go to this link:
4.5.2. Carbonate Hardness
.
Return to Temperature, pH, KH and GH
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Dave says
In reply to Lachan ….. Most blackwater fish like rams need acid-neutral pH of less than 7.2 to spawn. I would go ahead and do a 95-100% water change with slightly remineralized RO/DI water.
lachan says
Dave,
I’m looking into possible reasons my pair of rams would have eaten the batches of eggs they produced. They readily spawn in clean water at the right temperature, but in about 24 hours they consume the eggs. My pH is around 8.2 and my water is moderately hard at 6.14 dGH.
The rams are currently on vacation in a larger tank until their smaller tank clears back up from a mishap, but I would like to have one successful spawn from them when they return.
Would you recommend attempting to lower dGH first before the arduous task of attaining lower stable pH?
I have an RODI unit, but I only use it to top off, never to fill after a water change when that is needed.
Dave says
In reply to Fabiano …. I’ve never had a problem with mixing the more peaceful Lake Malawi’s (peacocks and Labs) with Lake Tanganyikans, even with low GH/KH water.
Fabiano says
Thank you for your blog. Do you think that fishes from lake Tanganyka are also quite adaptible as the cichlids from lake Malawi in aquariuns with pH around 7 and low GH? There are some youtubers who claim that we should not keep these two groups of cichlids together in the same tank.