
Carbonate hardness (KH) is important for nitrifying bacteria. For every 10 ppm of ammonia converted to nitrate very roughly 71.4 ppm (71.4 KH or 4 dKH) of carbonate is used up. Each gram of most ammonium salts requires very roughly three grams of sodium bicarbonate (baking soda), A reference is useful: “How Alkalinity Affects Nitrification”, Barillo, 2015
“During nitrification, 7.14 mg of alkalinity as CaCO3 is destroyed for every milligram of ammonium ions oxidized. Lack of carbonate alkalinity will stop nitrification. In addition, nitrification is pH-sensitive and rates of nitrification will decline significantly at pH values below 6.8. Therefore, it is important to maintain an adequate alkalinity in the aeration tank to provide pH stability and also to provide inorganic carbon for nitrifiers.”
Note this industry standard of 7.14. assumes the equation of:
4NH3 + 4CaCO3 + 7O2 + 4CO2 —> 4Ca(HCO3)2 + 4HNO3
It has to do with the species of carbonate. At a pH of 6.2 to 8.3 virtually all the carbonate in solution is actually bicarbonate per a complex graph called a “Bjerrum Plot”.
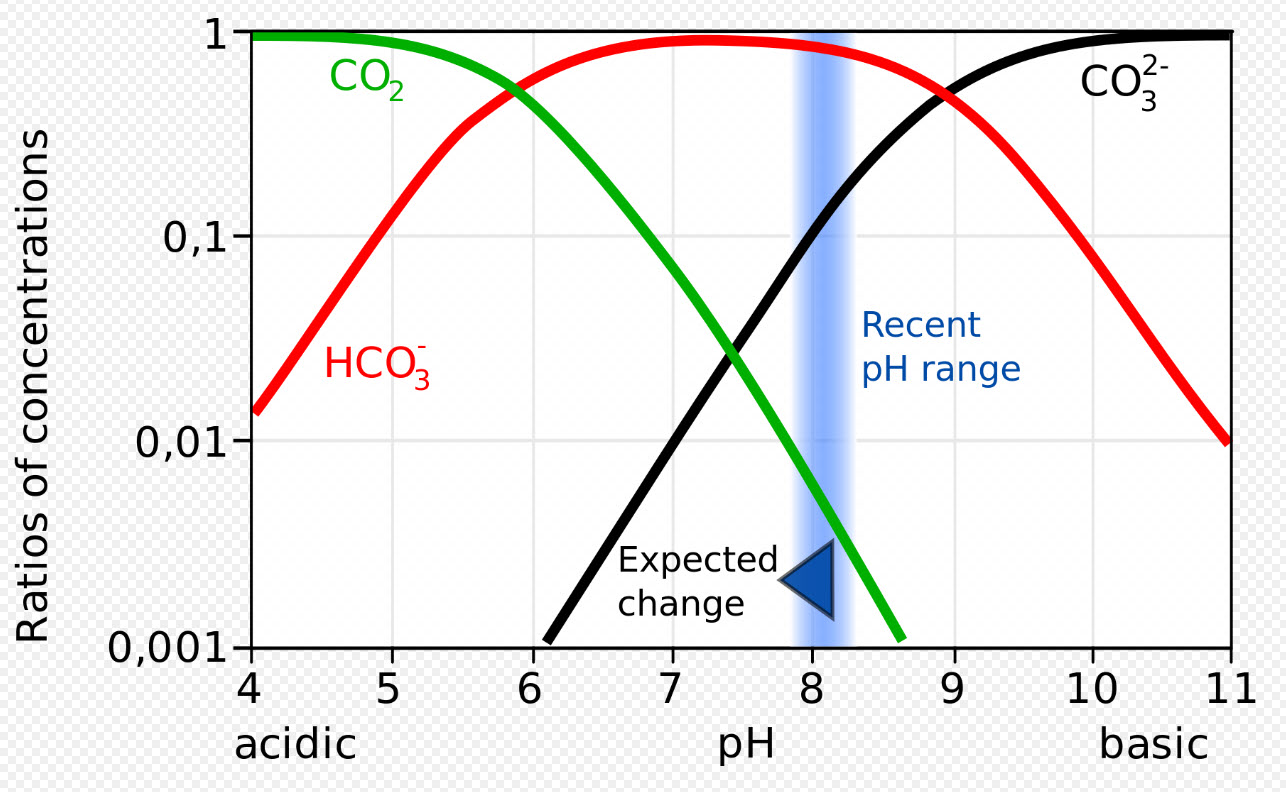
So driving calcium carbonate to bicarbonate is a one to one equation. And it looks only at the nitrogen in the ammonia as that is technically how test kits look at it. So the weight ratio become 100/14 (100 is the molecular weight of calcium bicarbonate and 14 is the molecular weight of nitrogen) or 7.14.
There is a lot of research which says bicarbonate (typically baking soda) needs to be constantly added to aquaculture systems in order to have good nitrification by bacteria. So when one is cycling one needs to constantly add sodium bicarbonate powder unless one has high KH water to begin with.

I have used baking soda (sodium bicarbonate) to raise the pH for many years. Be cautious when adding baking soda to a fish in cycling. You want to slowly add baking soda to maintain a pH of 7.4 to 7.8, no higher. Higher pH can result in an ammonia pulse.
It is also very beneficial to add crushed coral to the filter. When cycling I monitor the pH and keep it above 7.4 with sodium bicarbonate. It is sometimes surprising how much sodium bicarbonate is needed to do that.

Note that this position is a change as of 08/07/2022. I had previously had a severe case of confirmational bias which said KH was unimportant during cycling, even though I was adding tons of baking soda every time I cycled a tank. Confirmational bias is real!
.
Return to Temperature, pH, KH and GH
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!

Brad - UK says
Hi Dave,
Just so I am clear, having a good KH during cycling is important as every 10 ppm of ammonia converted to nitrate very roughly 71.4 ppm (71.4 KH or 4 dKH) of carbonate is used up.
So what happens once. your tank is cycled? I am struggling to understand why the KH is not used up as a matter of daily ammonia to nitrate conversion.
I hope that question makes sense!
Thanks,
Brad
Dave says
In reply to K …. Based on your experience I rewrote this page to go back to sodium bicarbonate. It is just a whole lot safer.
K says
I am 3 weeks into a fish-in cycle and pH has been about 6.6. Your fish-in cycling page says to add 1 tablespoon of baking soda per 10 gallons, but I decided to add a sodium carbonate instead since this page says it’s better. Big mistake. I only added 1 tablespoon to 36 gallons, but within minutes a fish died and the rest were not swimming right. I checked the pH and it was >8.8 (as high as it goes) so I frantically started pumping out water and pouring more in but couldn’t get the pH to come down AT ALL, even with what seemed like a 100% water change (pumping and pouring at the same time so can’t say for sure). I even tested the pH of the water before I poured it in thinking this seemed impossible and it was 7. I ended up adding 30ml of vinegar and the pH immediately came down to 7. Should I not have used the sodium carbonate in place of baking soda or did I just use way too much? Thanks!
Dave says
In reply to Tom … You are correct. Keep a mesh bag and toss in a corner of the tank
Tom says
How might one add crushed coral to a tank filtered only with an UGF? Keep a mesh bag and toss in a corner of the tank? It would seem of limited use to mix it in with the gravel as you point out that it needs replacing every few months.
Dave says
In reply to anonymous …. Whoops. Thank you for the correction.
Anonymous says
For every ten ppm of ammonia converted to nitrate very roughly 4 KH (71.4 ppm or dKH)
Should it be
For every ten ppm of ammonia converted to nitrate very roughly 4 dKH (71.4 ppm or KH) ?