
The going mantra is that tropical fish require “stability” and “constancy” of pH and temperature. Supposedly any rapid change over 0.2 pH or 2 degrees temperature will “shock”, “stress” and possibly kill the fish. This idea is a myth. Experimentation and scientific research by the author and universities has shown this to be patently false. This article elucidates some of that experimentation and research.
Note this is a very long and boring article only for the real nerds among us, like the author.

Test of The Myth of “Rapid Changes in Water Parameters will Kill Fish”
I had four Malawi fish aquariums. They are all very heavily stocked with no rocks and few plastic plants. All the tanks were on a drip water changes system with hard 8.2 pH well water and are kept at 76 degrees F. They were all very well aerated. They were all extremely “over filtered” both with powerhead undergravel filters and large K1 fluidized bed sumps.
I set up a simple experiment with these four heavily stocked aquariums. I took two of the tanks off the drip system (hard well water) and set up a “garbage can conditioning” acidification system, where the water change water for these two tanks was RO water brought to 6.5 pH and 70 TDS with hydrochloric acid and salt. The two aquariums that were no longer on the drip water change system were then brought to 6.5 pH and kept at that pH for roughly six months.

One of the acid tanks and one of the drip tanks was then raised in temperature to 83 degrees. The other two tanks were allowed to drop to whatever my room temperature gave. It turned out my room temperature (typically 69 degrees) gave a stable 72 degrees in the two well aerated tanks.
I then conducted a very informal test where I simply netted about 30 to 50 fish and moved them randomly around the four tanks. I would do this at least once a day, every time I was around the tanks with nothing to do. I might move ten fish from tank one to tank two, ten fish from tank four to tank three, ten fish from tank two to tank four, and ten fish from tank three to tank one.
This netting and plunking was quite easy in the overstocked tanks with few or no decorations. The overstocking that prevented any territorial aggression from occurring in any of the tanks also made netting and moving fish easy.
The netting and moving was simply “net and plunk” with no “conditioning” at all. This was a temperature shock of 83 to 72 or 72 to 83, and a pH shock of 6.5 to 8.2 or 8.2 to 6.5, all in random combinations. This was literally thousands of fish that were netted and plunked over a six month span. NONE of the fish died or even seem to be phased by the moves. NONE. ZERO! And note Lake Malawi is known to have a very stable temperature and pH.

What is Seen in Nature
Anyone who has ever taken a swim in a pond or a lake in the afternoon will tell you the water temperature at the surface can be considerably different from the water temperature just a few feet down.
Any biologist familiar with freshwater lakes will tell you the pH and temperature of the water changes constantly and amazingly quickly from one layer to another layer in the water. When scientists attempt to measure pH in a lake or pond with a probe they have some big problems. As the probe descends the pH can change up to two points in just a few feet. And the changes are unpredictable.
The pH in natural bodies of water changes rapidly as sunlight interacts with carbon dioxide and algae. Carbon dioxide in water is acidic. The carbon dioxide content of water varies constantly. The acidity goes up and down rapidly:
“On a bright sunny day, in ponds with low alkalinity (less than 50 mg/l calcium carbonate) and intense phytoplankton blooms, pH may rise from a morning value of 7.0 to an afternoon peak of 9 or 10 due to photosynthetic removal of carbon dioxide from the water.”
(Masser, Texas A&M University, 2012).
When the water at the surface of a lake is 9 pH and 85 degrees the water five feet down can be 7 pH and 75 degrees (Wurts and Durborow, University of Kentucky, 1992). And fish constantly go up and down through these thermoclines with no ill effects.

In the book “Limnology” by Wetzel, a study was done on a somewhat alkaline pond that was fifty meters in length. At 2:00 PM on a sunny day the pH varied very radically across the length of the pond, going from 7.6 to 10 in as little as two meters. And fish were swimming rapidly across these waters for the entire study with no apparent effect.
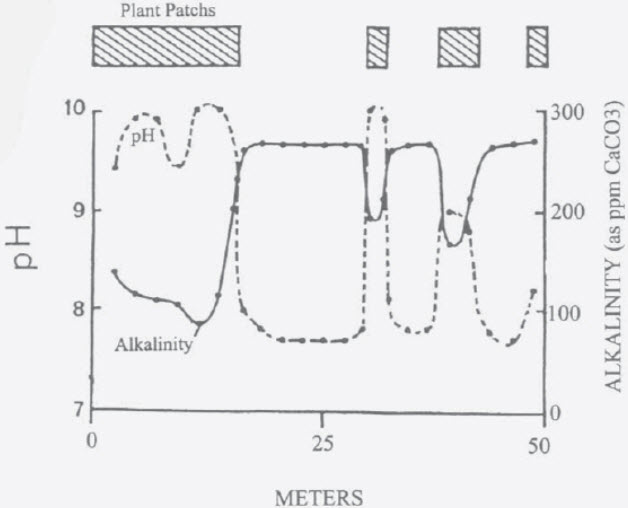
The alkalinity was going from 4 KH to 14 KH at the same time. This is very typical in nature and presents no problems with the fish in the ponds.
In the paper “Phytoplankton Photosynthesis, Micronutrient Interactions and Inorganic Carbon Availability in a Soft Water Vermont Lake”, Allen, 1972, the pH went from 5.7 at 10:00 am to 9.6 at 12:00 noon in an acid water lake.
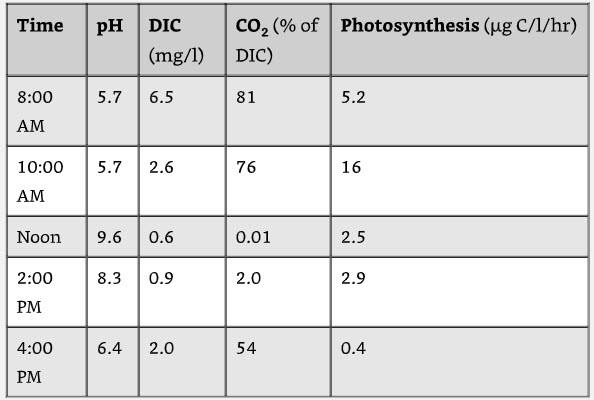
This rapid change had no effect on the fish in the Lake.

In the Amazon, during the flooded season, these differences are much larger, going from 5 to 9 pH and 80 to 95 degrees in a few feet. Fish like corydoras constantly swim up and down and through these large pH and temperature differences. Fish like corydoras are NOT a “sensitive” fish.
In an alkaline lake such as Lake Malawi the pH changes are somewhat buffered and muted by the carbonate salts, but the temperature changes are not muted.
This is from an article: “Recirculating Aquaculture Tank Production Systems”, Masser et. al. Southern Regional Aquaculture Center, March 1992
“Fish generally can tolerate a pH range from 6 to 9.5, although a rapid pH change of 2 units or more is harmful, especially to fry”,
“A rapid change of 2 units or more” is TEN TIMES the 0.2 units that many hobbyists ascribe to.
What people fail to recognize is how effective the skin and gills of the fish are in preventing “shock” from occurring. Acid water is rich in something called the “hydrogen ion” (H+1). Alkaline waters are rich in something called the “hydroxy ion” (OH-1). The gills and skin of a fish have something called a “bilipid membrane”.
Bilipid membranes are good at passing pure unchanged gases such as oxygen and carbon dioxide through the membrane. They are VERY good at PREVENTING charged ions from passing through the membrane. “Hydrogen ions” and “hydroxy ions” are charged ions and cannot pass through the membranes of the gills or skin. So pH changes cannot thus affect the fish.

University Research on Temperature and pH shock in Fish
One obscure but pertinent reference is in the article “Saprolegniasis (Winter Fungus) and Branchiomycosis of Commercially Cultured Channel Catfish”, Durborow et. al., 2003
“In experimental trials, rapid decreases in water temperature (72o F down to 54o F, or 22o C down to 12o C, in 24 hours) have been shown to impair the fish’s immune system, cause a loss of mucus from the skin, and temporarily suppress mucus production by goblet cells in the dermal layers of the skin”
So an 18 degree F. (10 degree C) drop in temperature caused some relatively minor temporary changes in the fish (note it didn’t kill them!). That is right, 18 DEGREES of change caused minor changes which might be described as “shock”! This is not the 2 degree sensitivity being claimed by all the “experts” of social media.

Another Research Paper is “Evaluation of pH Shock on Hatchery-reared Rainbow Trout”, Witschi et. al. 2011
“The effect of transferring hatchery-reared rainbow trout (Salmo gairdneri) from water with a pH of 7.2 to water with pH’s ranging from 8.5 to 10.0 was evaluated in 48-h tests. All fish survived in the control (pH 7.2) and at pH 8.5. Survival was 88% at pH 9.0, 68% at pH 9.5, and O at pH 10.0. After the 48-h exposure, the remaining test fish were fed their usual pelleted food. Trout in the control and those held at pH 8.5 fed well. Only a few of the fish held at pH 9.0, and none of those held at pH 9.5, fed.”
So going instantaneously from 7.2 pH to 8.5 pH did not affect rainbow trout. This is a 1.3 pH change, with no effect. That is correct, a 1.3 CHANGE IN pH CAUSED NO PROBLEMS. And rainbow trout are a known sensitive fish.

Researchers can measure the amount of a substance called “Heat Shock Proteins” (HSPs) in the internal organs of fish. Measurable levels of these proteins indicate an increase in the stress levels of a fish. University researchers found sudden upward temperature increases created measurable amounts of HSP at the following levels: 14 degrees F (Airaksinen et. al. 1998), 22 degrees F (Basu N. et. al. 2001), 27 degrees F (Curries S. et. al. 2000).
This 14, 22 and 27 degrees F is hardly the 2 degrees F many hobbyists seem to think is life threatening to a tropical fish. And while the proteins went up, none of the fish died or had any severe problems due to the radical temperature differences.
There was some old research (Wiebe, 1931) where several species of fish were taken from lakes with a pH of 4.4 to 6.4 and put in lakes with pH of 8.2 to 8.7. Several species living in the high pH lakes were then transferred to the lower pH lakes. The fish tolerated the extreme shifts with no mortality and no “shock”.

A Less than Perfect Study
Another paper used a common aquarium fish, the red bellied Pacu, to test sudden thermal and pH shock. The study was “Acceptance of Thermal and pH Shock on Red Belly Pacu (Piaractus brachypomus) in Adverse Rapid Environmental Conditions”, Yahiya et. al., 2020.
This study had no mortality when the Pacu went from 820 F (280C) to 730 F (230C), 640F (180C), 910F (330C), and 1000F (380C) very suddenly (“net and plop”). The fish exhibited some limited behavioral changes, but they dissipated relatively quickly. These are changes of 90F (50C) and 180F (100C) with no mortality or lasting effect. This is much more than the 20F claimed as a problem by some social media aquarium gurus.
This study also measured the difference in fish mortality when giving the Pacu pH “net and plop” changes from 8.0 pH to 7.0, 6.0, 9.0 and 10.0 pH. They got mortality with three of these four rapid changes. But there was a fundamental problem with the research which negated these results. The researchers put the Pacu suddenly into newly formulated water solutions which had a starting point of tap water that was hard 8.0 pH water with 1 ppm ammonia in it. They should have used RO or distilled water.

The researchers took Pacu which had been living in 8.0 pH water for ten days and plopped them into water at 9.0 and 10.0 pH. When they went up in pH they had a confounding factor. The ammonia became toxic. 1 ppm ammonia is not too much of a problem at 8.0 pH. But at 9.0 pH the ammonia is ten times more toxic, and it is at levels which are known to kill fish. Not surprisingly they got 40% mortality at 9.0 pH.
At 10.0 pH 1 ppm of ammonia is 100 times more toxic than at 8.0 pH and typically 100% fatal. These researchers not surprisingly had 100% mortality at 10.0 pH. The authors of the study attributed the mortality to the sudden pH change. That conclusion was unwarranted.
The authors also tested putting the Pacu rapidly into water which was much lower in pH than the 8.0 pH water they had been living in for ten days. At 6.0 pH (a 2 point change in pH) the Pacu had a 20% mortality and were very stressed (panting etc.). At a pH of 7.0 there was little stress and no mortality.
But these results have another confounding factor. The tap water used had a high carbonate content. When you acidify a high carbonate content you release large amounts of carbon dioxide. Large amounts of carbon dioxide are deadly to fish (AND the fish “pant”). The authors of the study attributed the mortality to the pH. Again, that conclusion was unwarranted.

The authors of this study looked at behavioral changes such as panting, congregating on the bottom, feeding behavior and “piping”. Again, the ammonia and carbon dioxide confounding factors could have given the same results. So the conclusions of the behavioral part of the pH study were not warranted.
In any case, even if the conclusions of the study were warranted, the huge changes made in both temperature and pH were MUCH larger than the 2 degrees F and 0.2 pH changes claimed by social media “experts” to be damaging to fish.
.
Return to Water Stability is not Important Page
Return to Temperature, pH, KH and GH
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Dave says
In reply to Mark B …. Fish are kind of funny with regard to ammonia output. They only put out a LOT of ammonia for several hours after they have eaten. And the fish were only in the bag 45 minutes. So you could easily get zero ammonia output in that small of a time frame.
Mark B says
Hi Dave, some anecdotal evidence here for this chapter (4.8.1. Rapid Thermal and pH Shifts)
We picked up an angelfish today from a LFS. It was in an 87 degree F tank with angels and discus. I wrapped the fish bag with a winter coat, and my wife held it in her lap on the way home. 45 minutes later – cut, pour, plop into the QT – which was at 75.9 degrees F and a pH of 7.6. The pH of the LFS water in the bag was 6.2. So, a temp swing of 11 degrees and pH swing of 1.4 and the angelfish was fine – eagerly up at the front glass looking for a hand to feed him – just like in the store.
One question: My test of the LFS water showed 0 ammonia, 0 nitrites. How is that possible at a pH of 6.2? During the rapid cycling tests I saw how cycling stops below a pH of 6.5.
Thank you, Mark