
We are going to predicate this article with a warning:
.
The ammonia level per the API test should always be 0.25 or lower, not because ammonia is that toxic, but rather because any ammonia indicates poor biofiltration which will kill fish from excess bacteria in the water.
.
If one has a new aquarium under six months old and one sees an increase in ammonia above 0.25 ppm per the liquid tests like API, this is generally nothing to worry about. Random ammonia spikes happen all the time with new aquarium setups. But if the increase is still there a week later, there is something wrong in the biofiltration of the aquarium and it needs to be addressed. To understand this better go to this link:
5.2.3. Ammonia Spikes
If you want to become very bored and delve into a pretty pointless dissertation only for real nerds like the author, read on.

Toxicity of Ammonia
Most of the “ammonia” found in the aquarium is actually something called “ammonium” or “ionized ammonia (NH4+). Ammonium is relatively harmless to fish. But some of the ammonium (NH4+) converts to “un-ionized” ammonia (NH3). “Un-ionized”, “free” or gaseous ammonia (NH3) is somewhat toxic at levels greater than 0.05 ppm. Technically un-ionized ammonia is 100 to 400 time more toxic than ammonium, which only comes into play at low pHs. We ignore the much lower toxicity of ammonium below in order to simplify the discussion and the math.
The toxicity levels of un-ionized ammonia are best shown by the popular Seachem test kit which measures the free ammonia levels rather then the total ammonia (TAN or ammonia+ammonium) levels of the popular API test kit:
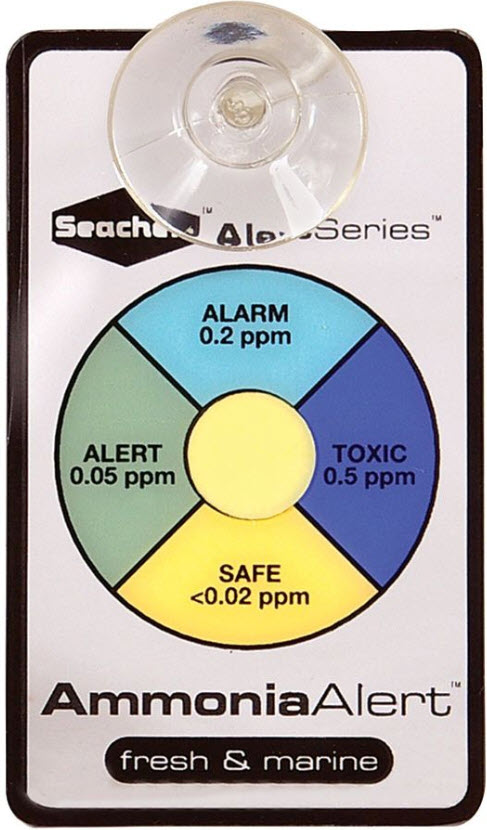
The “ALERT 0.05 ppm”, “ALARM 0.2 ppm”, and “TOXIC 0.5 ppm” are for free ammonia (NH3) and are levels which are very accurate per the research literature. Again, this Seachem test is for free ammonia gas (only NH3), not total ammonia.
Most hobbyists use a liquid test called the API ammonia test kit. The API test kit measures total ammonia nitrogen (NH3/NH4+, ammonia+ammonium), not free ammonia gas. This is a big difference in the two tests and the source of much confusion, to put it mildly. At 7.0 pH and 20 degrees C. the Seachem test above converts to 8 ppm “ALERT” level, 32 pm “ALARM” level and 80 ppm “TOXIC” level.
This is the API test:

The most accurate way to illustrate the chronic toxicity per the API test of TAN (un-ionized ammonia+ionized ammonium) is a chart derived from the University of Florida ammonia toxicity chart (Publication #FA16, “Ammonia in Aquatic Systems”, Ruth Francis-Floyd, 2009) which references aquariums and aquaculture in tanks. This reference states:
.
“Un-ionized ammonia (UIA) is about 100 times more toxic to fish than ionized ammonia. This UIA toxicity begins as low as 0.05 mg/L”
.
This statement is the guideline that has guided fishkeepers for many years and it is VERY accurate per many reference papers. If this 0.05 unionized ammonia chronic toxicity level is looked at with reference to pH at 28 degrees C (82 degrees F.) it becomes the “Alert”, level of the Seachem alert dot test below. The Seachem test also give the following “Alarm” and “Toxic” levels at 28 degrees C:
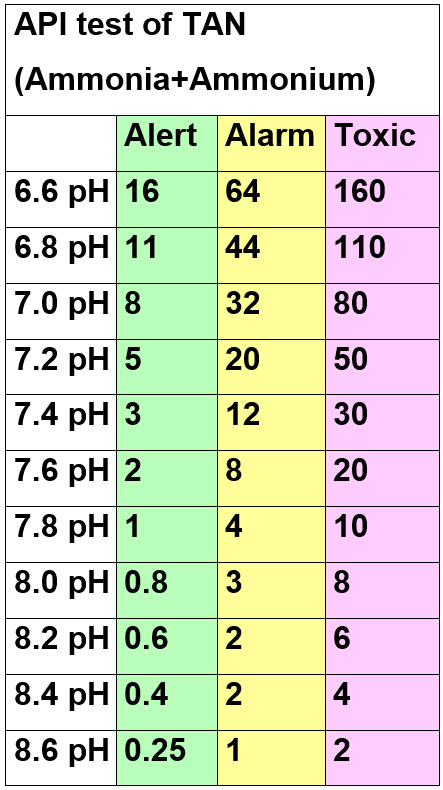
These numbers will shock most hobbyists but they are accurate as confirmed by multiple papers delineated below. Note that this is also per Seachem, who many hobbyists seem to think is the most expert supplier in the world. Dark green on the API ammonia+ammonium test kit only becomes toxic at pHs higher than 8.0 per the Seachem data. Most well meaning but ill-informed commentators on social media will tell you anything over 1 ppm ammonia is toxic to fish.

Another set of numbers for ammonia is given by the Website of the German Ammonia Testing Kit made by Sera.
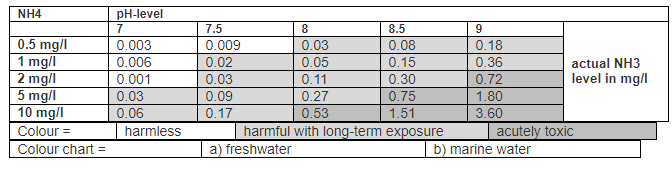
This says that at a pH of 7.0 and 25 degrees C an API test kit reading of 8 ppm ammonia+ammonium is harmful with long term exposure. A level of 8 ammonia+ammonium with the API test kit at a pH of 7 is NOT acutely toxic. More importantly the toxic level for ammonia gas can be seen to be about very roughly 0.4 ppm.
At a pH of 7.0 and a temperature of 25 degrees C. (77 degrees F), 0.566% of the total ammonia nitrogen (TAN) is present as free ammonia (Florida Department of Environmental Protection, Chemical Laboratory Methods Manual, “Calculation of Un-Ionized Ammonia in Freshwater”, 2001 and several other references). In aquariums we measure total ammonia (TAN or ammonia+ammonium). So the roughly 0.4 must be multiplied by 177 (100/0.566), giving 70 ppm ammonia+ammonium. At a pH of 8.0 the multiplier is 15.

Research Pertinent to Ammonia Acute Toxicity
To illustrate the low acute toxicity of ammonia+ammonium, a research paper is pertinent (“Studies on the Toxicity of Ammonia, Nitrate and Their Mixtures to Guppy Fry”, Alan et al, 1977):
“Using guppy fry as the test fish the individual and joint toxicities of ammonia and nitrate were estimated in static tests at constant pH and temperature. The 72-h lc50 values were 199 and 1.26 mg 1−1 −N for potassium nitrate and free ammonia, respectively. The toxicities of mixtures of the two were additive except at very low ammonia to nitrate ratios.”
The ammonia toxicity of 1.26 here needs to be modified. This is for free ammonia gas (NH3). Ammonia exists in water in two forms: ammonia gas (NH3) and ammonium (NH4+). The aquarium test kit measure both forms of ammonia, or the Total Ammonia Nitrogen (TAN or ammonia+ammonium). Note that the pH of the test is irrelevant as the test measure un-ionized ammonia. The pH is only relevant if one is measuring ammonia+ammonium, such as in the API test.
At a pH of 7.0 and a temperature of 25 degrees C. (77 degrees F), 0.566% of the total ammonia nitrogen (TAN) is present as free ammonia (Florida Department of Environmental Protection, Chemical Laboratory Methods Manual, “Calculation of Un-Ionized Ammonia in Freshwater”, 2001 and several other references). In aquariums we measure total ammonia (TAN or ammonia+ammonium). So the 1.26 must be multiplied by 177 (100/0.566), giving 223 ppm ammonia+ammonium. This is extremely high. Much higher than I would have thought. It is close to three times the toxic level of the chart above. And it should be noted this is for guppy FRY, not adults.

Another study on the cichlid Tilapia is pertinent: (“The Acute Toxicity of Ammonia on Tilapia Larvae and Fingerlings”, Benl et.al. 2003,
“The aim of the present study was to determine median lethal concentrations (LC 50) of unionised ammonia (NH3) for the larvae and fingerlings of Tilapia (Oreochromis niloticus L. 1758). The mean 48 h LC50 values were determined as 1.009 ± 0.02 mg/l for larvae and 7.40 ± 0.01 mg/l for the fingerlings.”
Again the numbers have to be multiplied by 177. So the fish larvae had a limit of 179 ppm ammonia+ammonium and the fingerlings had a limit of 1,310 ppm ammonia+ammonium at 7.0 pH and 25 C. But of course this is Tilapia, which are known for being very resistant.

Trout are a much more sensitive fish. So let’s look at “Acute Toxicity of Ammonia and Nitrite to Cutthroat Trout Fry”, Thurston et. al., 2011
“The toxicity of ammonia and of nitrite was tested on cutthroat trout (Salmo clarki) fry (1-3 g) for periods up to a month in eight laboratory flow-through bioassays. Median lethal concentration (LC50) values for ammonia (mg/liter un-ionized NH3) were 0.5-0.8 for 96 hours, and 0.3-0.6 for 36 days. Nitrite LC50 values (mg/liter NO2-N) were 0.5-0.6 for 96 hours, and 0.4 for 36 days. Tissues of fish exposed for 29 days to 0.34 mg/liter un-ionized ammonia evidenced degenerative changes in gills, kidneys, and livers. Cutthroat trout fry are comparable to rainbow trout (Salmo gairdneri) fry in their susceptibility to acute toxicity from aqueous ammonia and nitrite”.
Multiplying by the 177 factor (0.3 X 177 = 53 0.6 x 177 = 106) gives an LC50 toxic ammonia+ammonium concentration of 53 ppm to 80 ppm 36 day toxicity ammonia+ammonium at 7.0 pH and 25 degree C. Again, this was for trout FRY.

Neolamprologus multifasciatus Tanganikan Shellie or “Multie”Per Hütter 1990:
Per Hütter 1990:
“The lethal dose for fish at about 1 mg/l ammonia (at 15° C), leading to suffocation with gill necrosis.”.
Again, in order to get the aquarium numbers per the API test, we must multiply these numbers by 177 (many hobbyists have looked at these numbers and mistakenly thought they were the same as what the API aquarium ammonia+ammonium test kits give). This gives 177 ppm ammonia+ammonium for acute toxicity at 7.0 pH and 25 C. Again, this is very high.

There are a whole series of journal articles on this subject. I won’t continue to bore you with the details. Instead I’ll just summarize it in a chart. Adding up all the research and references on the toxic level of ammonia one get the following chart for the TOXIC level, where 50% of the fish are dead after 48 to 78 hours:
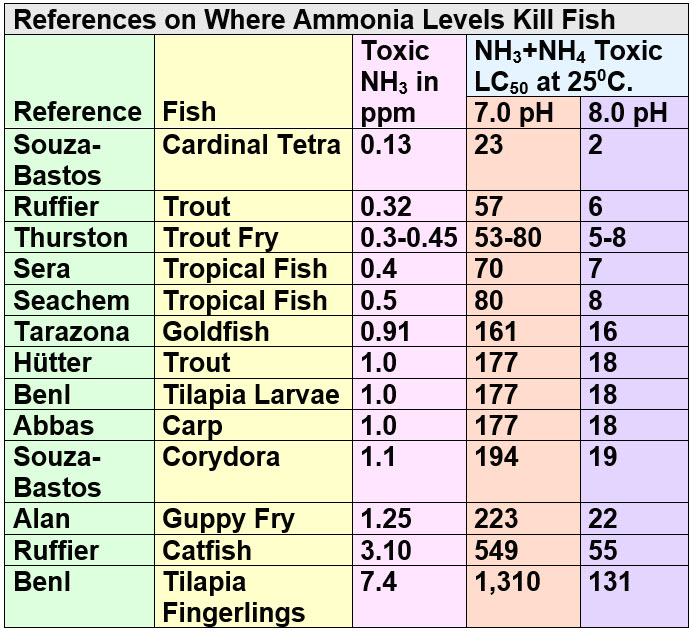
This is pretty firm data. Not exactly something one can argue about, although many will try. The stubbornness of the “ANY ammonia is toxic” crowd is astounding.
One critic of this section insisted not only that I was claiming “ammonia isn’t toxic at all to fishes,” but also that the chart is deliberately and disingenuously inaccurate since it uses 250 C. (770 F.) whereas the University of Florida data and the Seachem test use 280 C. (820 F.). He said the numbers change AND make ammonia “much more toxic”.
“Interesting”. Yes it is true that ammonia is more toxic at higher temperature. I used the 250 C. (770 F.) number simply because that was what some of the studies in the chart used, not realizing I was thus being inconsistent with what I had said well above. One has to multiply the last two columns by 0.82 (82%) to get the toxic numbers for 280 C. (820 F.). This is not exactly an earthshaking change. So the 23 becomes 19.
Now obviously a 50% death rate in two to four days is NOT saying that this level is safe for fish. This chart only establishes the level where you DEFINITELY WILL kill fish. The following analysis is where the exposure is long term or “chronic”.

Research on Chronic Toxicity of Ammonia
In the lower concentrations, ammonia can be detrimental to the long-term health of a fish. Per the EPA (1989):
“Lower concentrations of ammonia can cause a reduction in hatching success, reduction in growth rate and morphological development, and pathologic changes in tissues of gills, livers, and kidneys.”
These effects mean a fish will be more susceptible to pathogens for the rest of its life if it is exposed to ammonia+ammonium concentrations. But defining a “limit” on this concentration is difficult. It is very dependent on the pH of the aquarium water. Note this effect is also very species specific. Fish species found in ponds and lakes are generally much more resistant to ammonia than species in rivers.

The Sera chart above has a lower chronic exposure “harmful” limit of 0.02 ppm ammonia gas. This translates into 0.02 x 177 or 3.5 ppm chronic exposure “Alert” limit for ammonia+ ammonium for all fish at 7.0 pH and 25 degrees C.
Note that the going consensus in the aquarium community is that the level of ammonia gas (NH3) should be less than 0.05 ppm in a healthy aquarium (the “alert” level in the Seachem test). At a pH of 7.0 and a temperature of 250 C (770 F), this translates into 177 X 0.05 = 8.85 ppm ammonia+ammonium by the standard aquarium API test kit. At a pH of 8.0 and a temperature of 250 C (770 F), this translates into 0.89 ppm ammonia+ammonium by the standard API aquarium ammonia+ammonium test kit.

To illustrate the chronic toxicity of ammonia, a research paper is pertinent: (“Ammonia Toxicity, Tolerance, and Excretion”, Ip et al, 2002):
“Elevated ammonia levels in the environment are toxic. Temperature has only minor effects on ammonia toxicity expressed as total ammonia in water, and ionic strength of the water can influence ammonia toxicity, but pH has a very marked effect on toxicity. Acid waters ameliorate, whereas alkaline waters exacerbate ammonia toxicity. The threshold concentration of total ammonia ([NH3] + [NH4+]) resulting in unacceptable biological effects in freshwater, promulgated by the EPA (1998), is 3.48 mg N/liter at pH 6.5 and 0.25 mg N/liter at pH 9.0.”
The terms again need to be corrected to what is used in the hobby at 7.0 pH. 3.48 mg N/liter ammonia+ammonium at pH 6.5 translates into 0.18 ppm ammonia gas. 0.18 x 177 = 32 ppm ammonia+ammonium at 7.0 pH and 25 C. So the EPA upper limits for discharge of water into freshwater bodies is 32 ppm ammonia+ammonium at 7.0 pH and 25 C and 3.2 ppm ammonia+ammonium at 8.0 pH and 25 C. per the API test. This can be taken as a chronic toxicity “Alarm” level.

Tilapia were studied for long term exposure to ammonia. “Chronic ammonia toxicity to duckweed-fed tilapia”, Fatma et. al. 2004:
“The effects of prolonged exposure to sub-lethal un-ionised ammonia nitrogen (UIA-N) concentrations on the growth performance of juvenile Nile tilapia (Oreochromis niloticus) fed on fresh duckweed (Lemna gibba) grown on pretreated domestic sewage have been investigated. The experiment was conducted over 75 days using juveniles with a mean body weight of 20 g. This study concluded that, for raising Nile tilapia in fishponds fed on fresh duckweed or other feed, the toxic level of UIA-N and its negative effect on the growth performance lies between 0.07 and 0.14 mg UIA-N l−1. It is recommended that the UIA-N concentration be maintained below 0.1 mg l−1.”
These numbers need to be multiplied by 177. This means for Tile Tilapia the lower limit for negative long-term chronic effect for ammonia+ammonium at 7.0 pH and 25 degrees C. is 12 to 25 ppm for chronic exposure. But remember, Tilapia are known to be very hardy and resistant to ammonia.

Per Hütter 1990:
“The lethal dose for fish at about 1 mg/l ammonia (at 15° C), leading to suffocation with gill necrosis. 0.03 to 0.05 mg/l ammonia lead to chronic damage, in which trout are particularly sensitive”.
Again, in order to get the aquarium numbers per the API test, we must multiply these numbers by 177 (many hobbyists have looked at these numbers and mistakenly thought they were the same as what the API aquarium ammonia+ammonium test kits give). This gives 5.31 to 8.85 ppm ammonia+ammonium chronic long term toxicity at 7.0 pH and 25 C. Again, this is very high.

Per “Understanding Your Fish Pond Water Analysis Report”, Stone et. al., University of Arkansas, 2006, the long term “acceptable level” of un-ionized NH3-N is “less than 0.4 mg/l”. This translates into 0.4 x 177 or 71 ppm ammonia+ammonium at 7.0 pH and 25 degrees C. for chronic “Alert” level exposure. But this is for pond fish, which are resistant to ammonia.
This is much higher than the other references and illustrates the difficulty in interpreting this data. The whole term “Alert” is very open to interpretation so the numbers will vary a large amount. There are other articles on the subject . But again we won’t bore you with all the details. Condensing all the various “opinions” and numbers for ammonia+ammonium at 25 degrees C. one gets the following chart:
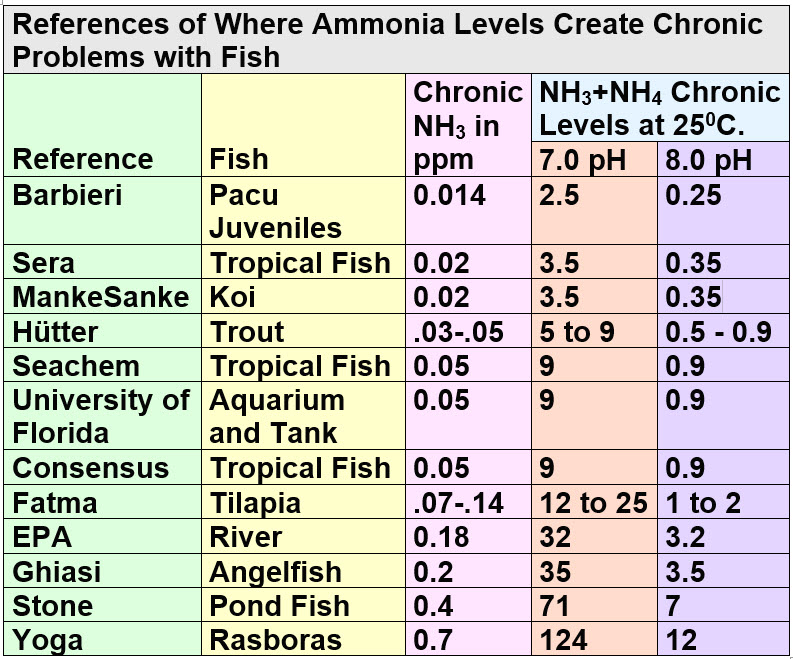
The Seachem number looks very good from this data. This is the number where on has to start testing regularly and monitoring things. It is the number where there can be long term health consequences for fish from ONLY the ammonia.

A VERY Important Caution
Now again note that ANY ammonia-ammonium is indicative of poor biofiltration. And poor biofiltration kills fish because of high bacteria counts in the water. So we do NOT recommend ignoring a reading of ammonia+ammonium over 0.25 (The API test can read 0.25 with distilled water) at ANY pH, especially if it lasts for more than a week.
This relationship is important when one looks at what people do when they have ammonia readings greater than 0.25 per the API test kit. All the advice in social media is along the lines of “do a 50% water change“. In actuality most of the time the advice should be to either, “stop cleaning your filter” or “add some good biofiltration“.
Note that the pre-eminent group which researches raising aquarium fish, the Ornamental Fish Research Group at the University of Florida, makes almost exactly the same caution. This is what they said:
.
“In healthy ponds and tanks, ammonia levels should always be ZERO. Presence of ammonia is an indication that the system is out of balance. Therefore, ANY ammonia in a pond or tank should alert the producer to start corrective measures.”
.
Note that the capitalized emphasis points were taken directly from the quote (Publication #FA16, “Ammonia in Aquatic Systems”, Ruth Francis-Floyd, 2009). This is almost word for word the same caution the author made at the beginning of this article.

Pathology Studies
There are another series of studies where the pathology of the gills were examined under a microscope and the point at which significant changes occurred was noted. These studies are:
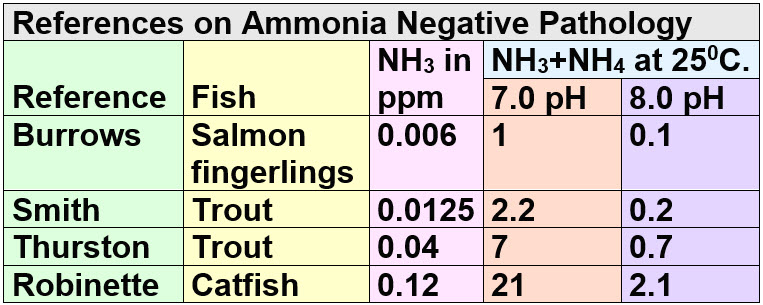
Now of course everyone who thinks ANY ammonia is poisonous will key in ONLY on the 0.1 ppm at 8.0 pH of Burrows and make that the ONLY study on ammonia toxicity. One study among many is not a “gold standard” and means little.
These studies look at LONG TERM pathology changes in fish. All these studies say that the changes seen will only slightly harm the health of the fish. I.e. these levels over the span of a few months will NOT kill your fish.
Indeed, pathology changes are common reactions to changes in the environment. Human livers change their pathology at detectable levels at one beer per day. But, again, we agree that any level of ammonia+ammonium greater than 0.25 ppm for more than a week or so needs to be addressed from the standpoint that something is wrong with the biofiltration.

Shipping Bags
In a bag of fish which has taken three to five days to get to its destination the carbon dioxide builds up in the water. And the oxygen level drops. And the acidity drops. And typically the temperature will drop or go too high. And the fish gets stressed when its swim bladder suddenly expands during take-off of the plane its riding in. Then the swim bladder is rapidly compressed during landing. The ammonia+ammonium levels in these bags can reach very high levels. Like 30 to 80 ppm ammonia+ammonium. All of these together can kill the fish.
While the low pH resulting from the carbon dioxide makes the unionized ammonia (ammonia gas) covert to relatively harmless ammonium, there is a limit to this effect. Very high ammonia level causes ammonia gas to diffuse across the gills of the fish into the fish’s bloodstream.
The ammonia gas is converted to ammonium in the bloodstream and cause the pH of the blood to rise. The internal organs begin to leak blood and hemorrhage. The body cavity fills with a weak solution of blood and a light maroon coloration becomes apparent in the body cavity and surrounding tissue. This will kill the fish.

These sort of ammonia concentrations are virtually impossible to get in a home aquarium. So, when someone says their aquarium fish have “ammonia burn” they generally are in truth dealing with bacteria hemorrhagic septicemia, not ammonia poisoning.
One constantly sees on social media things like: “My fish at at a pH of 7.8 with 1 ppm ammonia and they have red patches, what do I do?” . ALL the answers to this person will be along the lines of “You have ammonia poisoning, do a ton of water changes“. And ALL the answers will be wrong. At a pH of 7.8 ammonia will need to be over 10 ppm for red patches to appear. The fish have bacterial septicemia.

Even in shipping the ammonia alone probably wouldn’t kill the fish. But a whole host of things is going on in a shipping bag. This combination effect in shipping is shown by a journal article: (“Tolerance to Temperature, pH, Ammonia and Nitrite in Cardinal Tetra, Paracheirodon axelrodi, an Amazonian Ornamental Fish”,OliveiraI et. al., 2004); ;
“Poor water quality condition has been pointed out as one of the major causes for the high mortality of ornamental fishes exported from the state of Amazonas, Brazil. The purpose of the current study was to define water quality standards for cardinal tetra (Paracheirodon axelrodi), by establishing the lower and higher for lethal temperature (LT50), lethal concentration (LC50) for total ammonia and nitrite and LC50 for acid and alkaline pH. According to the findings, cardinal tetra is rather tolerant to high temperature (33.3 oC), to a wide pH range (acid pH=2.9 and alkaline pH=8.8) and to high total ammonia concentration (23.7 mg/L). However, temperatures below 19.6 oC and nitrite concentrations above 1.1 mg/L NO2- may compromise fish survival especially during long shipment abroad.”
Note this reference says that cardinal tetras, a fish known for its sensitivity, survives 23.7 ppm of ammonia+ammonium (TAN, the API test), if ammonia is the only factor to consider. This is astounding! Note this only says they survived, so the lethal level for cardinal can be MUCH higher. Here is the data from this study:
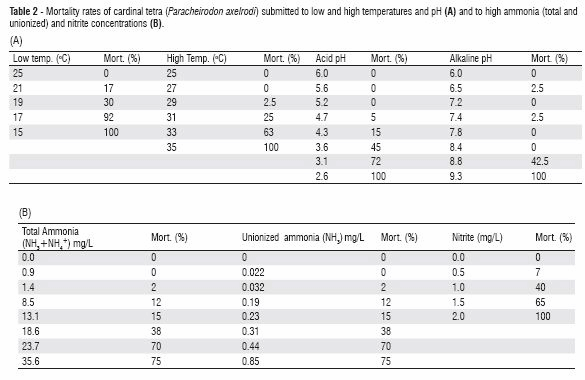
This takes some interpretation. The test was run at 6.5 pH. Converting the numbers to 7.0 pH requires some math. At a ammonia+ammonium of roughly 10 ppm at a pH of 7.0 there started being significant short-term acute toxicity to cardinals. But this study looked at multiple variables in a way which makes it very difficult to come up with any firm number for ONLY ammonia+ammonium.

Symptoms of Ammonia Problems
Many times it is reported that fish are repeatedly ramming the sides of an aquarium. This is typically because of some toxin in the water. One of these toxins can be ammonia:
“Sublethal concentrations of ammonia can cause hyperexcitability, resulting in fish crashing into the sides of the aquarium in response to any disturbance” (McKenzie et al. 2008; Olson and Fromm 1971)
So any time there is a lot of glass ramming by fish I check the ammonia and nitrite levels. If there is zero ammonia and nitrite I begin looking at things like water changes with water which has chloramines.

Ammonia and pH Relationship References
Free ammonia gas is directly related via a logarithmic relationship to pH. At a pH of 8.0 the free ammonia at a given level of total ammonia (TAN) is ten times higher than at a pH of 7.0. Since free ammonia is the poisonous part of the TAN, this is significant and must be considered.
This pH relativity is shown by this Blue Ridge Fish Hatchery’s chart:
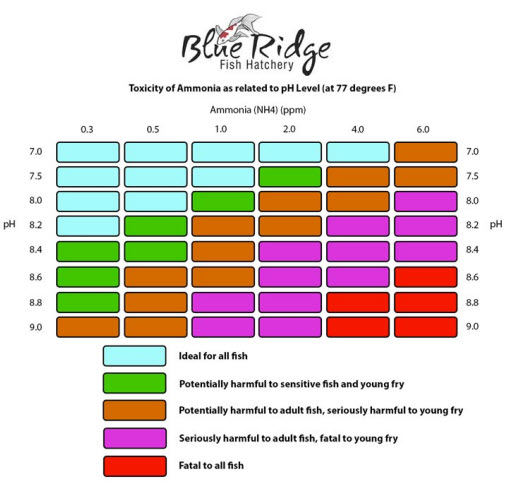
This chart says pond fish only start getting a negative effect (and “alert” level) at very roughly 5.0 ppm ammonia+ammonium at 7.0 pH (77 degrees F, 25 degrees C.). At 8.0 the alert level is 1, the alarm level is 2.0 and the toxic level is 6.0. This is right in line with what was stated in the chart above.

A similar chart is as follows;
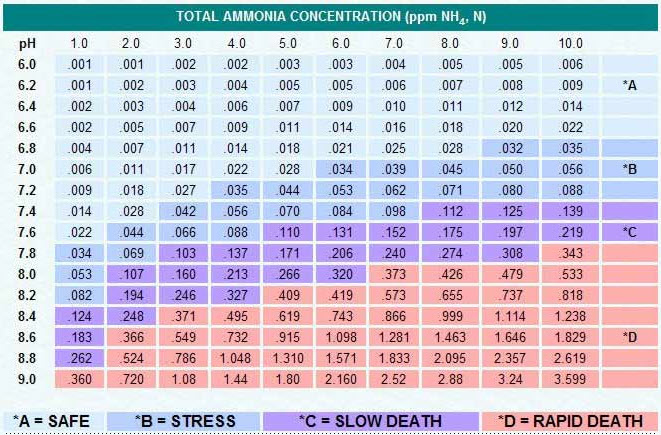
This chart says that at 7.0 pH fish start getting “alert” at 0.039 level of unionized ammonia. The “multiplier” to go from unionized ammonia to total ammonia is 1.77 at a pH of 7.0. This is 0.039 x 177 = 6 as the “alert” level of ammonia+ammonium at 7.0 pH. At 8.0 pH the multiplier is 15 so the alert level is 0.6, the alarm level is 1.5 and the toxic level is 5.5. Again, roughly right in line with the above chart of all the various references.
There are many versions of this chart that can be found in a whole series of fishery articles written for farmers with ponds. All these charts show that ammonia simply is not as toxic as the well meaning but ill informed commentators on social media say it is. But one has to temper these charts with the knowledge they are all for fish in farm ponds. The fish found in ponds are typically much more resistant to ammonia than say a discus from the Amazon.
Again, having said any hobbyist should strive to keep ammonia at the undetectable level. Not because ammonia is that toxic but rather because any ammonia is indicative of poor biofiltration. And poor biofiltration will give high bacteria counts in the water, which is bad for the fish.

.
Return to Water Parameters Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Dave says
In reply to Lee ….. Depends on the “soil”. Something like Aquasoil will release nitrogen in the form of ammonia. And any decomposing organic matter, including organic soils, will absorb and then release ammonium ions. It is called “cation exchange capacity” or “CEC”. But in a fertilized planted tank it is of little use. And understand, the soil only absorbs to the level in the water, say 6 ppm in the water will drop to 5 ppm in the water and in the soil when the soil absorbs some of the ammonia. Then when plants absorb 2 ppm from the water, the soil will release 1 ppm and both soil and water will be at 4 ppm. It is an equilibrium. The importance of CEC is vastly over-rated in the planted tank community. And the CEC equilibrium is of no use at all in a fish only aquarium, where you want ammonia to be zero.
Lee says
Hello, Dave. I’ve heard in the community that aquarium soil chemically adsorbs ammonium and can release it when it reaches its limit. Is this really true?
Through this wonderful website, I realized that most of the knowledge I knew was wrong. then I’ve made a lot of changes to my aquarium and I’m happy to have a successful result. Thank you, Dave.