
We are going to predicate this article with a warning:
.
The ammonia level per the API test should always be 0.25 or lower, not because ammonia is that toxic, but rather because any ammonia indicates poor biofiltration which will kill fish from excess bacteria in the water.
.
If one has a new aquarium under four months old and one sees an increase in ammonia above 0.25 ppm per the liquid tests like API, this is generally nothing to worry about. Random ammonia spikes happen all the time with new aquarium setups. But if the increase is still there a week later, there is something wrong in the biofiltration of the aquarium and it needs to be addressed. To understand this better go to this link:
5.2.3. Ammonia Spikes
Ammonia in the Aquarium
Fish excrete ammonia gas across the gill membrane to rid themselves of nitrogenous waste. “Ammonia” in water exists in two forms: un-ionized ammonia gas dissolved in water (NH3) and ammonium ions (NH4+). Un-ionized gaseous ammonia is toxic to fish at very low levels, while ionized ammonium is relatively harmless. The ratio of the two types of ammonia in water is directly related to the water pH and temperature. As pH decreases so does the portion of the ammonia that is in the toxic, un-ionized gaseous form.
The popular API test measures BOTH the ammonia gas and the ammonium ion. This is called “total ammonia nitrogen”, TAN or ammonia+ammonium. So one needs to look at the pH of the water when trying to determine the toxicity of ammonia as measured by the API test. The toxicity of total ammonia is ten times greater at a pH of 8.0 than it is at a pH of 7.0. This relationship is the source of a great deal of confusion in the hobby. Over and over one will hear “ammonia kills at anything over 1 ppm“. And this simply isn’t true IF one is talking about the API ammonia+ammonium test results.

Toxicity of Ammonia
The most accurate way to illustrate the toxicity of ammonia is a chart derived from the University of Florida ammonia toxicity chart (Publication #FA16, “Ammonia in Aquatic Systems”, Ruth Francis-Floyd).
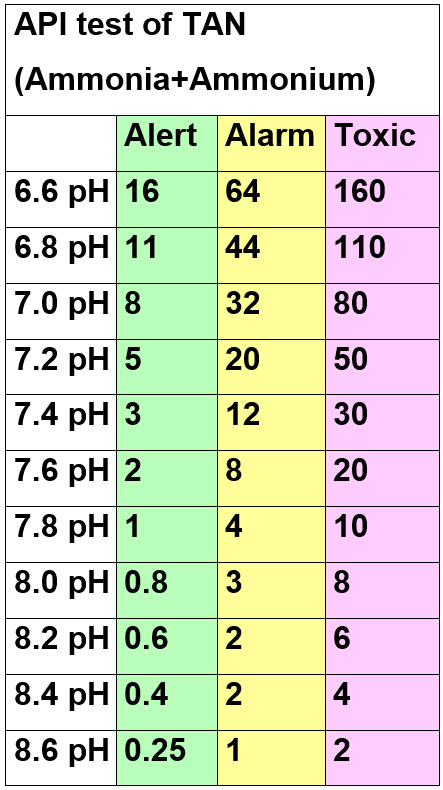
To read the ammonia+ammonium levels on this chart find the pH of the aquarium first. Then find that pH in the left column. Read across as to the levels the API ammonia+ammonium tests will show. Green is the “Alert” level where increased testing is called for. Yellow is the “Alarm” level where one should do a 50% water change. And “Toxic” is the level where one should do at least a 75% water change. Note many of these levels require diluting the aquarium water with 9 parts of distilled water. Test the diluted water. Multiply the results by ten and you have the levels in the aquarium.
These numbers will shock most hobbyists but these numbers are confirmed by many research papers by many universities so they are solid. Note that this is also per Seachem, who many hobbyists seem to think is the most knowledgeable supplier in the world.
Having said that one SHOULD keep ammonia+ammonium at the “undetectable level”, not because of ammonia+ammonium toxicity, but rather because it is an indicator of the health of the aquarium. Note that the API ammonia+ammonium test will measure 0.25 ppm (very light green) even with distilled water.
So “undetectable level” actually means equal to or less than 0.25 ammonia+ammonium as measured by the API test. An aquarium which has ammonia+ammonium at an “undetectable level” is probably a healthy aquarium with low bacterial counts. Low bacterial count is always the chief goal in aquarium water. Low ammonia+ammonium and low bacteria count typically go hand in hand in any established aquarium.
This toxicity of ammonia+ammonium is supported by a host of references from the aquaculture farmers. They are raising fish for profit and sickly or dead fish aren’t profitable. So their research tends to be very accurate. This research can be found at the following link:
5.2.1. Ammonia Toxicity in Depth
.

Ammonia Myths
There are some common misconceptions about ammonia+ammonium poisoning. One is that a malfunctioning automatic vacation fish feeder killed a bunch of fish through ammonia+ammonium poisoning. It wasn’t the ammonia+ammonium from the excess food that killed the fish. It was the bacteria and the bacterial toxins produced by the rotting food that killed the fish.
Many common bacteria (Streptococci and Clostridium to name two) produce toxins. These bacteria can reproduce rapidly. When some human eats spoiled meat and dies, death is not from the ammonia, death is from bacteria and bacterial toxins. The excess food rots and produces the bacteria and the bacterial toxins. The same holds true for when a fish dies and decomposes in an aquarium.

Many think ammonia toxicity is manifested by red patches on the skin. This is not true. The first sign of high ammonia is mad dashing of the fish around the tank. The fish sense the high ammonia and are trying desperately to get out of the bad water. With very high levels of ammonia the internal organs start to bleed and a dark maroon patch can be seen in the belly. Only VERY high levels of ammonia result in red patches on the skin. Most red patches on the skin are bacterial hemorrhagic septicemia, not ammonia poisoning.
Ammonia in More Depth
There are some aquarium hobbyists who are interested in delving deep into the science and the calculations behind all aspects of the hobby. For those who are so inclined the following is pertinent:
5.2.1. Ammonia in Depth
5.2.3. Ammonia Spikes
.
Return to Water Parameters Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

George says
First of all I would like to thank again you for your great article!
I would like also to point here that for the past 4 years API ammonia+ammonium test is giving me a measure of 0.25 ammonia as a result throwing a lot of money on buying several ” seachem magic liquids ” unfortunately and without any progress on my problem!
Furthermore before a week i changed half of filter material (seachem matrix with pot scrubbers ) and before I reach that article i was worried!
To sum up i would like to thank you again and to mention that the protein film on the surface has disappeared i think due to filte material and i will NOT worried about 0.25 ammonia measured by api test!
Regards George
Dave says
In reply to JL …. the table was created from a table by the University of Florida so I do not know the reason for the apparent discrepancy. I think the temperature was 70 degrees Fahrenheit.
JL says
I’m also curious about the numbers in the chart. Taking the 7ph for example at 32ppm ammonia and assuming a temperature of 79F (thus applying a factor of 0.006 per the study), the resulting UIA would be 0.19ppm. From my understanding, gill damage starts at 0.05ppm, and yet this concentration is only labeled as alert. Am I missing something?
JL says
Wondering what temperature that table is created for. Thanks.
Dave says
In reply to Loi …. The ratio of ammonium to ammonia is a constant for any given pH. So yes, some will convert back.
Loi Nguyen says
Hi, in the context of fish keeping, if the levels of ammonium in the aquarium decrease due to factors like plants absorbing it, will a portion of ammonia naturally convert back to ammonium to maintain a stable ammonia-ammonium ratio?