
Synopsis
The simple conclusion of this article is simply that no less than TEN tests done by three qualified scientists conclusively show that Seachem Prime does NO “detoxification” or “binding” of ammonia, not even on a temporary basis. Seachem has ZERO tests which prove Prime detoxifies ammonia. That is correct, ZERO tests.
Seachem also claims Prime and Safe detoxify nitrite and nitrate. The article then goes on to show that Prime does not detoxify nitrite or nitrate with more testing.
This article is very long and very boring and only for the real nerds like the author.
In More Depth
Prime and Safe are two water conditioners made by Seachem which make a series of false marketing claims for the products.
Note that I admit I am decidedly biased against the Seachem Company. I can’t help it. The amount of “pseudoscientific bedazzlement” they put out on ALL their products is just nauseating. They mix real science in with simply impossible “snake oil” claims in a manner which is very misleading to “newbies” in the hobby. And they have threatened to sue me for “libel”. I do not like to be threatened.
This article examines this particular false claim by Seachem:
.
False claim # 1, Prime and Safe “Detoxify” Ammonia, Nitrite, and Nitrate.
.
The Prime bottles say:
“Detoxifies Ammonia, Nitrite & Nitrate”
The Safe bottle says:
“Removes Chlorine, Chloramine, and Ammonia, Detoxifies Nitrite and Nitrate”
The Technician on the Seachem Test site say about fish in cycling:
“Dose Prime every 48 hours as needed to detoxify the ammonia in the tank, and keep the fish safe”.
And you will find that a good 90% of social media absolutely believes this to be good advice. Be forewarned that you will find yourself in the minority if you are publicly skeptical of this advice. This 90% of social media is wrong. And 90% of the people on social media who read this article will not change their mind at all about Seachem Prime. They either will not believe the testing described below or they will find a perceived fault with the testing which, in their minds, invalidates the testing.

Ammonia – Seachem’s Pseudoscientific Bedazzlement
The Seachem website does some, well, let us just say “amusing”, comments when it comes to their explanation of what Prime does to ammonia. If one wants a good laugh, read on.
Seachem claimed Prime “neutralizes” ammonia by converting it to what was called “imidium”. From Seachem Tech support page on 7/13/2018:
“Seachem Tech Support MS: Thanks for the question. Prime converts ammonia into a complexed imidium salt. This salt is a nitrogenous compound that can still be utilized by aerobic bacteria. In the same way they consume ammonia, these bacteria consume the imidium salt and release nitrite as a byproduct.”
From the Seachem Tech support page on 11/09/2011
“Prime converts ammonia into a complexed imidium salt. This salt is a nitrogenous compound that can still be utilized by aerobic bacteria. In the same way they consume ammonia, these bacteria consume the imidium salt and release nitrite as a byproduct. Unfortunately, while we have researched it extensively in our laboratory, I do not have any documents that I can provide you as proof. However, if you wanted to test this, it would be very simple and could be done by treating tap water containing chloramine with Prime and adding it to an established aquarium. Also add a Seachem Ammonia Alert to give continuous free ammonia readings. Prime will break the chloramine bond, leaving behind ammonia. Unlike other water conditioners it will then bind to the ammonia, producing a non-toxic imidium salt.”

The poisonous ammonia gas was supposedly converted to this “imidium”. This “imidium” was supposedly stable for 24 to 48 hours during which time the biofilters would supposedly remove it.
“Imidium” does not exist, it is a fictional chemical species invented by some marketer. Google “imidium”! You will find nothing!
Note that AFTER this website and this comment was posted on social media Seachem changed the name from “imidium” to “iminium”. From the Seachem question and answer forum one month after the comment above (about there not being any such thing as “imidium”) was posted to social media:
.
Last edited by Tech Support RT; 04-23-2019, 08:58. Reason: “Imidium” is a typo. The actual salt is “iminium salt“
.
Interesting. Over a span of seven years Seachem made the same “typo” at least five times.

Interesting! OK, so what is Iminium? From Wikipedia:
An iminium cation in organic chemistry is a functional group with the general structure [R1R2C=NR3R4]+.[1] They are common in synthetic chemistry and biology.
So iminium is one nitrogen atom with three radicals attached (radicals are basically any chemical other than hydrogen). This is a very instructive point. Ammonia gas has three hydrogen atoms attached to one nitrogen atom. So to convert ammonia gas to iminium requires adding three radicals (including one double bonded carbon) to the nitrogen atom.
At this point it is instructive to note the author is a degreed professional chemist. So this is his area of expertise.
Adding one radical to ammonia gas creates something called an amine. To do this one needs an environment with no water, a very high temperature, a very high pressure and something called metallocene catalysts. Adding three radicals to the nitrogen would require many more steps in many more outrageous types of chemical reactors. So creating an iminium molecule in an aquarium from ammonia is simply completely impossible. The creative writers at Seachem strike again!

One can see the process that occurred. Seachem became aware that their “imidium” claim had been exposed. So someone in Seachem Marketing Googled “imidium” and Google redirected them to “iminium”, an actual chemical species. So the marketer simply said that “imidium” was a typo and they actually meant to say “iminium”. They knew most people would lack the chemical training to know Seachem was knowingly making a false claim.
Now if you go on social media you will find there are many, many hobbyists who believe Seachem can do no wrong. These hobbyists will absolutely believe that Seachem has developed a chemistry capable of tying up ammonia as “iminium”. After all, Seachem is an honest outfit that never lies.
Here’s an even better deal: There’s a white pill I sell which is guaranteed to raise I.Q. by 40 points, make one irresistible to the opposite sex, and takes 20 years off your apparent age. It is only $998 per tablet. Just ignore the letters B-A-Y-E-R on the tablets. Honest! Would I lie?

Discussion for Ammonia Detoxification
In another article we prove beyond any shadow of a doubt that both Prime and Safe are 100% a chemical called sodium dithionite (Na2S2O4). Sodium dithionite is a very well studied chemical that has been use for many things in the past two hundred years or so. The chemistry of sodium dithionite is very well known. It is simply a weak reducing agent. And a reducing agent can do NOTHING to ammonia. That is simply a well established fact of science. It is NOT DEBATABLE, although many diehards will try.
Prime and Safe cannot “detoxify or remove ammonia”. The only way to truly “detoxify or remove ammonia” is to oxidize it to nitrate. And Prime and Safe are both the opposite of oxidizing agents, they are reducing agents. One chemical cannot be both an oxidizing and a reducing agent.
If a chemical reduces the pH of the water dramatically it can “detoxify” ammonia (actually it just converts ammonia gas to ammonium ions, which are relatively harmless to the fish). But Seachem says their products do not affect the pH and sodium dithionite does not significantly affect pH.

Note that some claim that Safe and Prime have a chemical called sodium bisulfate in them and that the sodium bisulfate neutralizes ammonia. They even reference papers on how poultry emissions of ammonia are reduced by using sodium bisulfate salt.
Sodium bisulfate is something called an acid salt. An acid salt simply reduces the pH of the water. And reducing pH will reduce the toxicity of the ammonia present. This is the mechanism that is used in poultry farming. But Prime and Safe do not reduce the pH. Even Seachem says they do not reduce the pH. And no sodium bisulfate was detected by the Raman spectrometer. And the amount of Prime and Safe used in an aquarium is so small that significant pH reduction would not be obtained even if the two products were 100% sodium bisulfate.

Creating Ammonium
When chloramine dissociates into ammonia gas and chlorine gas it is true that the ammonia gas is present for one billionth of a second or so before most of it combines with water to form ammonium ions. But it’s not the product doing the “neutralizing”, it’s just water doing what water does. The ammonium ion is much less toxic than the ammonia gas molecule.
Depending on the acidity the ammonia gas will be 0.001% to about 15% of the total ammonia/ammonium amount in microseconds. The ammonia and the ammonium typically will be converted to nitrate in most established biological filters in 2 to 24 hours.
Note that prior to 2015 Seachem was claiming that Prime was responsible for converting ammonia gas to “ionized NH4+ ammonia” (ammonium). Here is what their tech said:
“Prime does not function in the same way as many other ammonia removal aids/ ammonia reducing agents. Prime works to bind the harmful NH3 form of ammonia into ionized NH4+ ammonia.
When using certain test kits it is possible to get a false positive reading for toxic ammonia. The total ammonia value can be correct, as Prime will not create any ammonia that is not actually present or will not cause elevated concentration levels. Most conventional test kits are testing for total ammonia only and do not have any way to differentiate between forms. If Prime converts all of the free ammonia to ionized ammonia, the ammonia is no longer present in the toxic form, but can still be detected as “total” ammonia on a test kit.“
So Seachem went from “imidium” (2011) to “ionized ammonia”(2015) to “imidium” (2018) to “iminium” (2019). One has to wonder what will be next.
The products which claim to “detoxify” ammonia rely mainly on the fact that chlorine is 100 to 1000 times more poisonous than ammonia and that most aquariums have established biofilters which can remove ammonia quite rapidly. They also rely on the fact that very few people have aquariums with a pH greater than 8.6, the pH level at which ammonia becomes very toxic. So, most aquarium owner’s fish aren’t stressed from the amount of ammonia present.

We decided to test the ammonia claims using real chemistry (the exact test RECOMMENDED by Seachem!) and not anecdotal information. Here is what we found:
Ammonia Test Abstract
A simple test was set up to measure toxic ammonia gas concentrations in water which had various conditioners added to it. The test used the Seachem Alert free ammonia test. This test measure only FREE AMMONIA, not the “Total ammonia” (free ammonia plus ammonium ions) of the API test.
The Seachem Alert test duplicates what the gills of a fish do. The thin membrane technology only allows free ammonia (i.e. ammonia gas) through the membrane, just like the gills of a fish. In order for the ammonia to be “detoxified”, even for the short term, the ammonia must be tied up in some form which prevents the ammonia gas from penetrating the membrane of the test. This exactly duplicates the gills of the fish, so it is a very valid test for “temporary ammonia toxicity”
The test clearly showed Seachem Prime and Seachem Safe did not do ANY detoxification of ammonia, even for the short term.

The Test Used for the Ammonia
There is one point that must be emphasized here:
.
This test is the very test recommended by Seachem for showing how well Prime and Safe “detoxify” ammonia.
.
This point cannot be over emphasized. What is interesting is that many on social media will go on and on about how the test doesn’t show the “detoxified” ammonia so it is invalid. If it is invalid why does Seachem recommend it?

We have to emphasis for all readers that the Seachem Alert test is completely different from the API ammonia test. This Seachem Alert test measures free ammonia (NH3). The API test measures total ammonia, or ammonium (NH4) plus free ammonia (NH3). Free ammonia will typically be only 0.1% to 1% of the amount of total ammonia. The Seachem Alert test duplicates the conditions in a fish’s gills. The yellow dot is a thin membrane which ONLY allows toxic free ammonia gas to pass through the membrane. So if Prime and Safe “detoxify” ammonia there should be a reading of zero on the yellow dots.
What is interesting is that from the comments about this article in social media will go on and on about how the Seachem Alert test doesn’t show the “free ammonia” so it is invalid. Exactly the opposite is true.
.
It’s apparent that due to marketing and the influence of what “everyone says,” a good 50% of readers of this article are so reflexively resistant to the facts that they simply will not see them. It’s easy to become entrenched in a position irrespective of the facts. We all do it. Science does its best to overcome this.
The test described here used a standardized amount of ammonia at a set pH and the results obtained with the Seachem Alert test were exactly what all the charts and graphs from the Universities predict. So the Seachem Alert test is accurate.
It appears that Seachem marketing and the resulting power of popular opinion has apparently blinded many hobbyists to this point. They will go on and on about how this Seachem test is not reliable. They do this because to admit that it is accurate would be admitting they are incorrect about the ability of Seachem Prime to detoxify ammonia. And their brain cannot let them make that admission.
.

Per Seachem Support Forum (YES you read that right, per SEACHEM!!!) this test will confirm that Prime and Safe “bind” and “detoxify” ammonia gas.
Seachem Tech Support MS, 11-09-2011, 18:23
Re: Prime questions…
Hello Troglodyte,
Thanks for the question. Prime converts ammonia into a complexed imidium salt. This salt is a nitrogenous compound that can still be utilized by aerobic bacteria. In the same way they consume ammonia, these bacteria consume the imidium salt and release nitrite as a byproduct. Prime will also bind with nitrite and nitrate, however, it will not prevent bacteria from consuming these compounds as well. Unfortunately, while we have researched it extensively in our laboratory, I do not have any documents that I can provide you as proof. However, if you wanted to test this, it would be very simple and could be done by treating tap water containing chloramine with Prime and adding it to an established aquarium. Also add a Seachem Ammonia Alert to give continuous free ammonia readings. Prime will break the chloramine bond, leaving behind ammonia. Unlike other water conditioners it will then bind to the ammonia, producing a non-toxic imidium salt. At this point you will see a 0.0 ammonia reading on the Ammonia Alert.
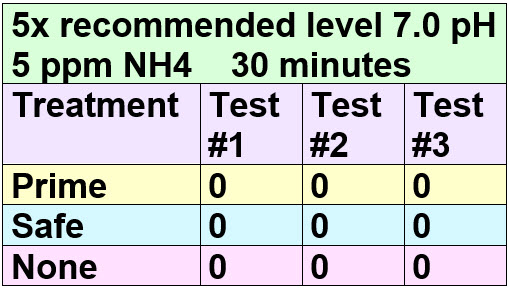
This claim by Seachem depends on the fact that most people have water around a 7.0 pH and at that pH a high level of ammonia (“Total ammonia” ammonium plus free ammonia) can exist but there will be a very low level of free ammonia. To test the “ammonia detoxifying” properties of Prime one HAS to use a high pH water.

Test Procedure
A gallon of distilled water was carefully brought to 8.0 pH by using baking soda (sodium bicarbonate). The gallon measured 8.0 pH after one week of the adjustment. This pH is important. Tap water with a pH of 8.0 is very common and that it is necessary to have this pH to get realistic test results. 50 ppm of total ammonia (the API test) is toxic at 7 pH while 5 ppm of ammonia per the API test is toxic at 8.0 pH.
If one did the test at 7.0 pH one would need to add ten times the amount of ammonia, or 50 ppm ammonia per the API test to get a toxic reading on the Seachem ammonia alert test. This amount of ammonia is not going to be seen in an aquarium so it is not a valid test. Only at 8.0 pH and 5 ppm of total ammonia (per the API test) do you have a valid test of Prime and Safe.
Note that this dependency of ammonia toxicity on pH is exactly what Seachem is counting on when they say to use their test to confirm Prime does indeed detoxify ammonia. Most people will test at roughly 7 pH and 5 ppm per the API test and come away convinced Prime does detoxify ammonia. That is simply not a valid test.

Ammonia in solution as “cleaning ammonia” (ammonium hydroxide) was added to 8.0 pH water to create a concentration of 5 ppm of total ammonia. Acid was then added to titrate back to 8.0 pH. Cleaning ammonia is a solution of the gaseous ammonia in water. When ammonia dissolves in water it forms two different chemical species. One species is the gas (NH3) and one is the ion (NH4)+1. The gas is poisonous while the ion is relatively harmless.
This level of 5 ppm “total ammonia” at an 8.0 pH should give about 0.26 ppm of gaseous “free” ammonia. Free ammonia is what harms the fish. Testing with several ammonia test kits (Tetra EasyStrips, Salifert Ammonia, Seachem total ammonia, API Ammonia test) confirmed the 5 ppm level of total ammonia. The pH was confirmed by three liquid test kits and two pH meters.
A series of nine bottles were filled with the ammonia water and a dosage of five times the recommended level of Prime was then added to three bottles and a dosage of five times the recommended level of Safe was then added to three more bottles. Three bottles, the “controls” had nothing added.
The bottles were then tested with the Seachem free ammonia test dots (a test kit version of the Seachem Ammonia Alert) using the 30 minute scale. This test tests for free ammonia gas, which is the chemical species which adversely affects fish.

Protomelas spilonotus Ovatus Mara Rocks
.
Results
The nine bottles were tested with the Seachem free ammonia test dots using the 30 minute scale. These were the results:
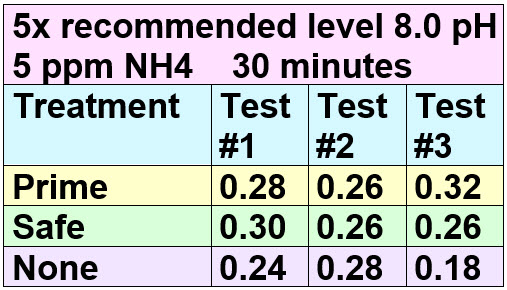
Prime and Safe failed to neutralize or detoxify ANY ammonia PER THE TEST RECOMMENDED BY SEACHEM! The 0.26 level of free ammonia was maintained, with no “complexing”, “detoxifying” or “binding” being done. So the two Seachem claims are simply marketing hype. Note 0.2 free ammonia gas is considered the “Alarm” level by Seachem, quite correctly.

.
Then, just to insure accuracy, the samples were allowed to sit for four hours and were retested:
Results
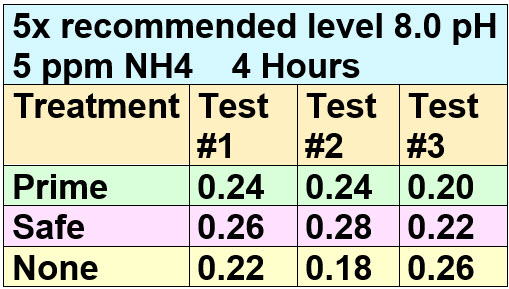
.
The test was then redone with 50 ppm total ammonia and 7.0 pH
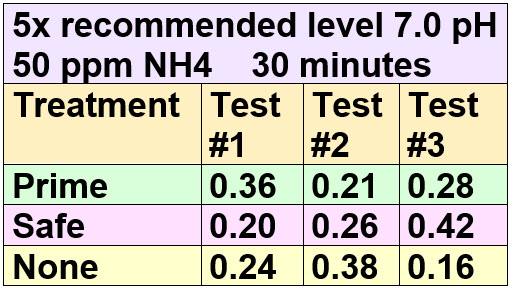
.
The test was then run again at 5 ppm total ammonia and 7.0 pH (note the Seachem dots do not do well with low concentrations. The human eye is just not able to pick up very light yellow colors)
This confirms that Prime and Safe do not have any time dependent neutralizing capabilities or pH dependent neutralizing capabilities. Note this is a very easy test anyone can do. Duplicate the test if you don’t believe it. The test only takes a few hours and about $40 in supplies. Two chemists did duplicate this test several times and confirmed the results of this test (see below). Another test using the API ammonia test kit was done by the author and also showed no effect on ammonia from Prime (see test ten below).
We have to reiterate that SEACHEM RECOMMENDS using their free ammonia dots to measure the detoxification effect their Prime and Safe product have. This is real hubris!

Tests that Others have Performed
Several threads were recently posted on the Reef2Reef blog on the reaction of Prime with ammonia ( https://www.reef2reef.com/threads/does-prime-actually-detoxify-free-ammonia-nh3.849985/page-8 ). Two chemists tested the ammonia neutralizing capabilities of Prime using far better testing methods than the author used. They both came to the FIRM, UNDEBATABLE conclusion that Prime does NOT detoxify or bind ammonia in ANY way.
Taricha was one poster who basically replicated my testing only in more depth. Taricha also did a test with living shrimp which showed they died from ammonia even with 5x the recommended dosage of Prime. She then replicated the test I did with chloramines and also found no ammonia detoxification with Prime and the ammonia from chloramines. Anther replication showed the test held over time. Here are her testing and conclusions:
.
August 5, 2021
This started when @Dan_P was looking at measuring NH3 with Seneye and was curious about performance near zero NH3. I suggested trying Prime to artificially zero out the NH3 sensor, and the results were weird… so I checked with my Seachem kit.
Prime by Seachem is commonly used to treat tap water, it dechlorinates Chlorine and Chloramine. This effect is strong and easily measurable by test kits.
But Prime also claims that it “…detoxifies ammonia. Prime® converts ammonia into a safe, non-toxic form that is readily removed by the tank’s biofilter.” They say that the normal dose of Prime can detoxify 1 ppm ammonia.
NH3 is the toxic form of ammonia, which under normal tank conditions is a tiny part of the total ammonia. Most chemical kits measure total ammonia – NH3+NH4, and so Seachem says that these kits can’t detect the effect of Prime to detoxify NH3. And one should instead use a test method that measures only free ammonia – NH3 instead, according to Seachem such as their “Alert” test and the small dot testers (MultiTest™ Ammonia kit).
“However, the best solution 😉 is to use our MultiTest™ Ammonia kit; it uses a gas exchange sensor system which is not affected by the presence of Prime® or other similar products. It also has the added advantage that it can detect the more dangerous free ammonia and distinguish it from total ammonia (total ammonia is both free ammonia and non-toxic ionized forms of ammonia).”
So here we go. I pulled a liter of tank water, spiked it with ammonia to ~1ppm total ammonia. API at 5 min confirms it’s in the ballpark of 0.5-1ppm total ammonia. Then I dosed a drop of Prime from two separate bottles (one new unopened) into the 1L of water. Approximately a double dose from each for a cumulative 4x dose of Prime and stirred.
After 30 minutes, I then used the ammonia sensing films from the Seachem kit to see if the measured free ammonia (NH3) was decreased by the “detoxifying” effect of Prime. The ammonia sensing discs are supposed to be read at 15 or 30 minutes to determine free ammonia.

- Each beaker has ~75mL of sample water.
- Bottom left is tank water only – clean zero
- Two in the middle top and bottom are replicates of tank water +1 ppm total ammonia – disks form a color as they should, approximately consistent with 1ppm total ammonia at ~8.0 pH (maybe around 0.05 on the top 30 minute scale of the color card)
- Top right beaker is tank water +1ppm total ammonia +4x dose of Prime – the disk forms exactly the same color as the samples that were not treated with Prime. The same amount of NH3 is apparently present.
So according to Seachem’s free ammonia kit, Seachem Prime does not do anything to decrease toxic free ammonia, NH3. If it has any effect, it’s gone within 30 minutes. (BTW, when I overdose prime to 30x recommended dose, it still didn’t decrease the NH3 measured.)

.
August 9, 2021
Amphipod test….
Amphipods are tough cookies. benthic organisms have a high ammonia tolerance. Marine amphipods have been found to have a lethal concentration of ammonia (to 50% of specimens) at 96 hours of anywhere from 0.83 to 3.39 mg/L of free ammonia, NH3 (not total ammonia).
4 treatments – 100mL bottles each with amphipods. Water mixed, dosed, and pH adjusted in separate 500mL containers, then exchanged into the amphipod bottles.
bottle 1
Tank water adjusted to pH 8.5-8.6
bottle 2
Tank water spiked with ammonia drops, adjusted to pH 8.5-8.6
bottle 3
Tank water spiked with ammonia drops and Prime in recommended amounts, adjusted to pH 8.5-8.6
bottle 4
Tank water with Prime only, same amount as bottle 3, adjusted to pH 8.5-8.6
two amphipods added to each bottle.
Ammonia levels will be increased from NH3 levels of 0, up through 0.5, 1.6, 2.7, and 4.4 ppm NH3 every 6-18 hours with the accompanying Prime additions to “detoxify” the ammonia (total ammonia levels 0, 4, 12, 20, 32 ppm.) Prime additions 1x dose for each ppm total ammonia. Prime will be added so each addition of Prime can “detoxify” all the ammonia in the sample, not just the last ammonia addition, in case previous Prime doses have stopped “detoxifying”.
The pH is elevated because the free ammonia needed to affect amphipods is so large, that higher pH is needed to do so with lower total ammonia. First column is the time in hours, the next 4 columns are the 4 treatments – Tank water with or without ammonia and with or without Prime, second to last column is the ammonia level at that time, last column is when and how much Prime was added.
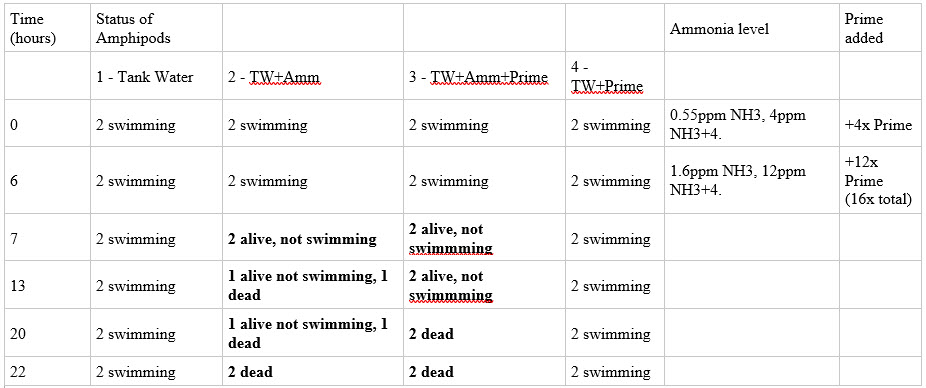
The clearly toxic effects began within an hour of moving to the second level of ammonia, so I did not go any higher.
The ammonia was clearly toxic with or without Prime added at 12x (for 12ppm ammonia). There was no detectable difference in the color of the Seachem disks, or the time when the amphipods could no longer swim, or any significant difference in the time when they were dead.
In the treatments with ammonia, without Prime the two amphipods were dead at 13 and 22 hours. With Prime the amphipods were both dead at 20 hours. There is no statistical difference in death rate with Prime as opposed to without Prime.
The amphipods not exposed to ammonia (with or without Prime) were able to swim throughout the process and were fed to a grateful yellow watchman goby at the conclusion.
Could the experiment be made more applicable and convincing with sensitive fish and a lower ammonia level? sure, but I have zero interest in doing that since it’s clear to me that Prime doesn’t detoxify ammonia and it would just end with dead fish.
This test is VERY damning. It used live creatures to prove beyond any shadow of a doubt that Prime does not “detoxify” ammonia. Let us emphasis this:
.
A Test with LIVING CREATURES Proved Seachem Prime DOES NOT DETOXIFY AMMONIA
.
What is interesting is that the three accounts on the Reef2Reef blog which were trying every debating trick and deception in the book to dissuade any and all that all this testing meant nothing were completely silent about this test. Interesting!

Taricha then did a third test on Prime using her water with chloramine
Here’s a quick and dirty Chloramine tap water check.
API Total ammonia test says my tap water chloramine registers as 0.4ppm total ammonia. (edit: This number may not be super accurate, I’ve never used total ammonia test to quantify chloramine ammonia, but it ought to be detected as easily as ammonia)

pH buffered to ~8.2. Top is tap water, middle is reacted with thiosulfate, and bottom is (duplicates) reacted with 2x dose of Prime (brand new bottle – opened this morning!)
Like in literally every other test – I see the dechlorinator. I see no evidence of a supposed ammonia binding ingredient.
.

.
Taricha then did another fourth test where she waited for a considerable length of time to see if the dots changed color with time:
Here’s three seachem disks in 4x dose of Prime with 2ppm ammonia, then one got moved down to 1ppm ammonia with 4 x prime, and one moved to a zero ammonia sample also with 4x prime. The third stayed in the 2ppm ammonia with 4x prime. (all were pH 8.1).
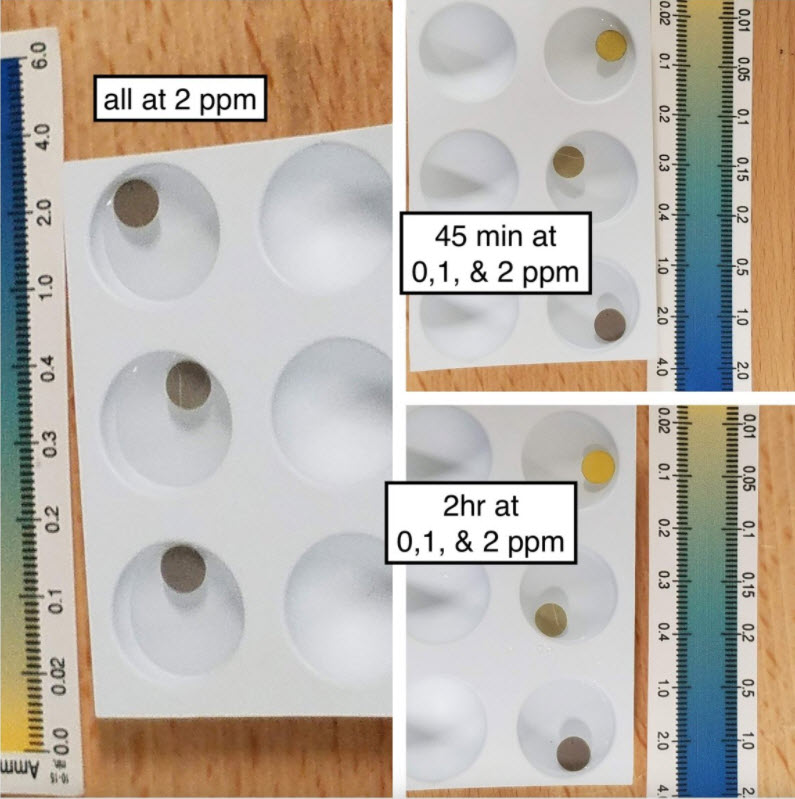
Do they take some time to fully reach equilibrium going down from high concentration? yep. But even with large doses of Prime they clearly respond to the removal of ammonia if it’s not there anymore, and after an hour or two the reading is stable. Substantial decreases in ammonia would be obvious.
Taricha then redid the tests with her tap water after buffering the pH. Basically just redoing the third test above. She got the same results in this fifth test.
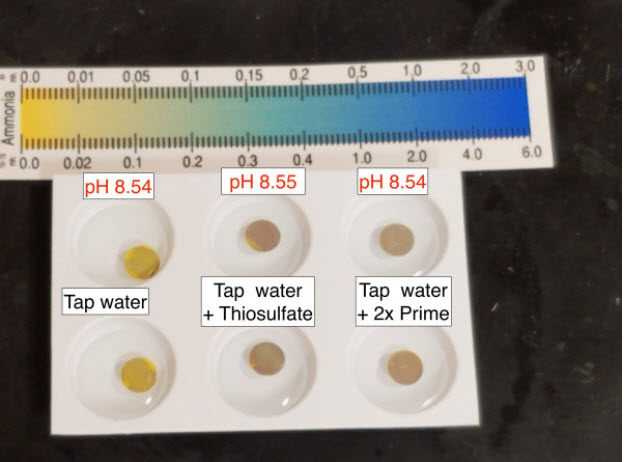
Taricha also noted:
Note that when @Dan_P specifically emailed Seachem requesting help understanding discrepancies between their claims about detoxifying ammonia and our measurements with NH3 sensing films, their response was essentially (my paraphrase) “Prime is very important to our business and please respect our right to tell you nothing about it.”
When I emailed Seachem asking them to explain why my testing showed what it showed, they did not respond.
Another member of this Forum, one Dan P, also replicated the authors test. Here is his results:
.
September 17, 2021
We also failed to detect Prime reducing free ammonia concentration with Seachem colorimetric films in their ammonia test kit and the ammonia alert badge. Seachem says these products detect free ammonia and can be used to observe Prime working. The conclusion is the same using Seneye or Seachem colorimetric films: Prime does not neutralize, combine with or detoxify ammonia in saltwater. And I will go out on a limb and say the similar claim about reducing nitrite and nitrate concentration is also nonsense.
I calibrated the Seachem badge and it does change color intensity with increasing free ammonia and in a linear manner. The Seachem ammonia alert badge does not detect any change in free ammonia concentration when Prime is added to artificial seawater containing ammonia.
The Seneye device which I also calibrated is another colorimetric free ammonia detecting film. It can detect lower concentrations of free ammonia than the Seachem ammonia alert badge and responds much more quickly. It too did not detect a change in free ammonia concentration when Prime was added to artificial seawater containing ammonia.

August 6, 2021
I examined the ability of Prime to reduce the amount of free ammonia with two different colorimetric films. One is contained in the Seneye device which also has a built in photometer to assess the color of the film and translate that into ppm of free ammonia. The second film is the Seachem Ammonia Alert badge. It too changes color as a function of the amount of ammonia in solution, but to convert color change to ppm of ammonia, the user is required to match the film color to reference color patches. To increase the sensitivity of ammonia measurement with the badge, I performed a color analysis on the film to detect color changes smaller than the eye can. The calibration curve below (open triangles) demonstrates how well the Seachem film color tracks free ammonia changes. We will discuss the orange filled circles on the plot next.
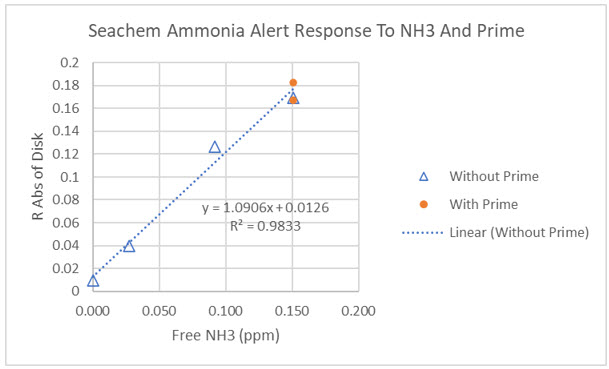
I repeated @taricha experiment, adding the Seachem ammonia sensing film 30 minutes after mixing aquarium water with ammonia (Hach multi parameter reference standard) and Prime. The film started to develop a color minutes after first being placed in this solution. The color was fully developed in 15-30 minutes. The two orange points on the calibration curve above correspond to the color analysis of the Seachem film 31 and 73 minutes after the film was placed in solution. Clearly, there was no detectable change in free ammonia.
The Seneye tells a similar story. When a dose of Prime is added to aquarium water spiked with ~0.5 total ammonia, there is only a very slight decline in free ammonia, but there is no dose response (see plot below). To test whether the Seneye film was affected by Prime, after one of the experiments, the ammonia level was changed and the film responded as expected.
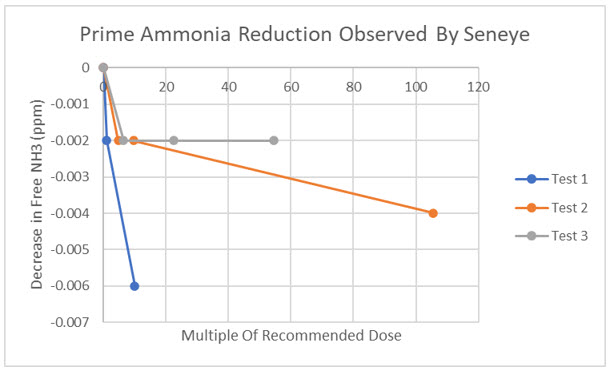
I have to conclude that our data, based on three different colorimetric films, makes a strong case for Prime doing little or nothing to reduce ammonia concentration.
Our data makes clear that Prime does not lower the concentration of ammonia in saltwater using Seachem’s own ammonia detection devices. We tried to make Prime work. We couldn’t.
Seachem never provided evidence that Prime could lower ammonia. They just said it did. Walking away from this product should be a no brainer.
These tests by Dan P were extremely well done and well thought out. They were much better than what the author did. And Dan P concluded that Seachem Prime did no “temporary detoxification” of ammonia. So the author’s tests of Seachem Prime and Safe were accurate per the well done testing of TWO other chemists.
.
This Makes TEN Separate Scientific Tests Which Concluded Prime Does Not “Temporarily Detox” Ammonia.
.
Despite repeated attempts by many hobbyists including myself to get ANY testing data from Seachem they have steadfastly refused to provide ANY testing data that refutes the tests done above. They have also steadfastly refused to explain why these tests were in error. I.e, the final score is:
.
Scientists did TEN tests that showed Seachem Prime does NOT detoxify ammonia
.
Seachem did ZERO tests that showed Seachem Prime does detoxify ammonia
.
Final Score 10 to 0
.
‘nough said.

Mikrogeophagus ramirezi, ram
Note that one very determined member of this Reef2Reef blog kept questioning all the testing done as not being valid (for no less than 23 pages of deflections and gibberish! 47 comments total!). Here is one of their responses:
“All good – but you still didn’t respond. The only question to me here is the following:
Seachem has a test developed – that they sell – for ammonia (free) – thats supposed to measure a decrease in free ammonia after Prime. There have been multiple studies like yours – over the years – including on @AquariumScience blog/website. If you research most or all of them – they have conflicting results from what Seachem says. So – something (I agree with you) is off. Its PROBABLY the method Seachem is using for testing as compared to the aquarium owner. But – as I’ve repeatedly said – you guys may very well be correct. The key word is detoxify. I do not believe that you have disproven that. (and except for anecdote, I do not believe Seachem has proven it). But I’m sorry – I dont get how you call out a company for ‘deceptive advertising’ – with your data. When you have not shown any reliable evidence that Prime does not detoxify (An in vivo claim) – with your testing. Seneye – also says – dont rely on ammonia levels with Prime added for 24 hours. So – who do we believe – Seneye? Seachem? Or your (IMHO – overly broad statements) – when you’re unwilling to do the testing required to prove what you’re saying. What you’re doing is inferring that Prime does ‘nothing’ for ammonia – because of ‘test kits’. All tests have confounding issues. Like I said – Call Seneye – Call Seachem – explain what you found – and ask for an answer.
IMHO – its a disservice to the ‘hobby’ to make a claim that ‘xxxx does nothing’ – when you’re not sure. Its a service to the hobby to try to verify claims made by manufacturers.”
This is simply garbage. Take for instance the quote “you have not shown any reliable evidence that Prime does not detoxify (An in vivo claim)”. Isn’t the term “in vivo” impressive? Sounds like this is a knowledgeable scientist. Just one problem. Understand, an “in vivo” test is a test done “inside a living animal” That is the definition of the Latin terms “in vivo“. But Seachem Prime is used outside the animal, in the water column. So there is no way to do an “in vivo” test. Whoops. Sounds good but it is gibberish.
Call me paranoid but I suspect this response is from the marketing department of Seachem. It is circular double talk. It is questioning the data in a way that seems to make sense but is actually completely … well… off the wall. It sounds very much like all the pseudoscientific bedazzlement one finds if one goes to the Seachem website.

Another commentator on this thread, a “Dr” with some 27 comments over 23 pages, simply mashed together a series of unrelated sentences that were one of three types of sentences:
- A response with a lot of scientific terms which were correct but didn’t apply to the situation at hand
- A response with a lot of scientific terms which were simply incorrect
- A response with a lot of scientific terms which were simply gibberish
This also sounds very much like all the pseudoscientific bedazzlement one finds if one goes to the Seachem website. Again, I suspect this is another person from Seachem, probably an actual chemist that works for them.

“A Brief History of Prime”
A series of “experiments” were done on the Aquarium Co-op blog titled “A Brief History of Prime” and published January 7, 2023. The core “experiments” used the API ammonia test kit (i.e. total ammonia and ammonium) to measure three conditions over time:
- A solution brought to 6.0 pH by acetic acid with ammonia and a 5X Prime dosage added
- A solution at 7.0 pH with only ammonia added (labeled the “control”)
- A solution brought to 11.0 pH by a saturated solution of sodium carbonate with ammonia and a 5X Prime dosage added.
The 6.0 pH solution went from green to yellow in four hours, the 7.0 solution stayed green and the 11.0 pH solution went from green to yellow over seven hours. The “experimenter” concluded this was “proof” that Prime did, with time, neutralize ammonia.

I politely commented that the 6.0 pH solution had the ammonia absorbed by bacteria which used the acetic acid as a source of carbon and energy to convert the ammonia and acetic acid to proteins and gaseous carbon dioxide. The saturated 11.0 solution had simply forced the ammonia out as gas due to the salt saturation.
I also pointed out that there were at least three more experiments needed to have a true experiment: 1, a 6.0 solution with no Prime; 2, a 7.0 solution with Prime and; 3, an 11.0 solution with no Prime.
I was immediately banned from the group and my comment removed. Ah, the power of Seachem. Note that it is my opinion the way this test was set up and the accompanying verbiage made it clear this was a professional chemist deliberately and knowingly misleading the reader. The use of acetic acid and sodium carbonate was not simply a coincidence.
Test 10 Repeating the “A Brief History of Prime” Test
We couldn’t resist repeating the test done by the author of “A Brief History of Prime”. The results were pretty conclusive that Prime did nothing to ammonia. This is the tenth test which proves exactly that.
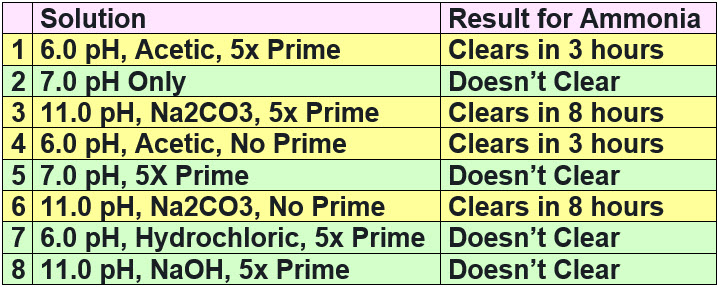
Experiments one through three simply repeated the test done by the “Brief History” author. Experiments four through eight showed Prime was NOT the reason for the ammonia clearing. Experiments seven and eight showed that the acetic acid and the sodium carbonate were responsible for the results obtained. This is an easy test anyone can do.

Nitrite and Nitrate Claims – Pseudoscientific Bedazzlement
We will now examine the nitrite and nitrate parts of the claims by Seachem about Prime and Safe. Seachem does a masterful job of deflecting any questions about how it’s Prime Products detoxifies nitrite and nitrate. This is from the Seachem website:
.
“How does Prime make a difference in reducing nitrates and nitrites?
A: The detoxification of nitrite and nitrate by Prime (when used at elevated levels) is not well understood from a mechanistic standpoint. The most likely explanation is that the nitrite and nitrate is removed in a manner similar to the way ammonia is removed; i.e. it is bound and held in a inert state until such time that bacteria in the biological filter are able to take a hold of it, break it apart and use it. Two other possible scenarios are reduction to nitrogen (N2) gas or conversion into a benign organic nitrogen compound.
I wish we had some more “concrete” explanation, but the end result is the same, it does actually detoxify nitrite and nitrate. This was unexpected chemically and thus initially we were not even aware of this, however we received numerous reports from customers stating that when they overdosed with Prime they were able to reduce or eliminate the high death rates they experienced when their nitrite and nitrate levels were high. We have received enough reports to date to ensure that this is no fluke and is in fact a verifiable function of the product.
.
This is pure snake oil claims. There is simply no such thing as “unexpected chemically”. The chemistry here is incredibly simple and cannot possibly be “unexpected chemically“. As a degreed chemist I find such comments to be downright insulting to the science of chemistry.

So they base this solely on anecdotal evidence, which is completely unreliable from a scientific point of view. Anecdotal evidence has never produced anything approaching a “verifiable function of the product“.
We decided to go ahead and do what Seachem somehow couldn’t do in its labs. We tested the claims that Prime and Safe neutralize nitrite and nitrate.
The various nitrite tests aren’t as accurate or “readable” as the Seachem free ammonia test. But if three tubes are filled with a solution of sodium nitrite and simultaneously tested, the tests can be easily be compared against each other as to color.

Three solution levels of sodium nitrite were prepared, 0.50 ppm, 1.0 ppm and 3 ppm. Three levels of Seachem Prime were then added to each of the three solution levels: No Prime (the “control”), the recommended dose of Prime, and three times the recommended dose of Prime. The control and the two levels of Prime were then simultaneously tested with various kits and the color compared. The following results were obtained:
API liquid Test
- 0.50 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 1.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended)were the same color
- 3.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended)were the same color
API test strips
- 0.50 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 1.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 3.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
Salifert Nitrite Test Kit
- 0.50 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 1.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 3.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color

Tetra Easy Strips
- 0.50 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 1.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 3.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
Capetsma 9 in 1 Aquarium trips
- 0.50 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 1.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 3.0 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
This show that nitrite is not removed by Prime. Now many will say Prime makes the nitrite harmless without affecting the test kits. If one believes this line of pure snake oil marketing hype, have at it.

Nitrate Test
The various nitrate tests aren’t as accurate or “readable” as the Seachem free ammonia test. But if three tubes are filled with a solution of potassium nitrate and simultaneously tested, the tests can be easily be compared against each other as to color.
Three solution levels of potassium nitrate were prepared, 20 ppm, 40 ppm and 80 ppm. Three levels of Seachem Prime were then added to each of the three solution levels: No Prime (the “control”), the recommended dose of Prime, and three times the recommended dose of Prime. The control and the two levels of Prime were then simultaneously tested with various kits and the color compared. The following results were obtained:
API liquid Test
- 20 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 40 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 80 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
API test strips
- 20 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 40 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 80 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
Salifert Nitrate Test Kit
- 20 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 40 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 80 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color

Tetra Easy Strips
- 20 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 40 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 80 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
Capetsma 9 in 1 Aquarium strips
- 20 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 40 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
- 80 ppm all the three concentrations of Prime (zero, recommended, and 3X recommended) were the same color
This show that nitrate is not removed by Prime. Now many will say Prime makes the nitrate harmless without affecting the test kits. If one believes this line of pure snake oil marketing hype, have at it.

Discussion for Nitrite and Nitrate Detoxification
In order to “detoxify” nitrite one needs to oxidize nitrite to nitrate. Once again, Prime and Safe cannot do that, they are not oxidizing agents.
And there is no chemical pathway by which nitrate can be made “nontoxic”, especially considering it isn’t that toxic in the first place. Nitrate can, under some very special anoxic conditions, be reduced to nitrogen gas by some species of bacteria. But is cannot be done using chemicals.

“But Prime Saved My Fish”
One recurring comment comes up whenever we post a comment on social media about how Prime does not even temporarily detoxifies ammonia. It is the “But Prime saved my fish” comment. The best way to address this comment is with another comment posted on the Reef2Reef blog by Taricha on August 8th, 2021. It is an excellent analysis.
.
Let me try to address a recurring question – “but Prime saved my fish from an ammonia event?”
Let me give a brief (haha) run-down of some reasons why a hobbyist might think that Prime etc has saved their livestock from ammonia when it hasn’t. (No judgment, less than a year ago, I had some fish loss and not much time, detected a little ammonia, and dosed Prime to “save” the unaffected fish. I was convinced it had worked. Here’s why I was wrong.)
1. Ammonia accumulates more slowly than we think. When I feed generously for my small fish, (pinch of flake + cube of mysis + cube of brine) I am only putting in enough protein to elevate total ammonia by 0.5 ppm.
If I feed a cube of mysis, a cube of brine, and a pinch of flake…
mysis: 3.3g x 7.6% protein x 16% N in protein = 40 mg N
brine: 3.3g x 3.7% protein x 16% N in protein = 20 mg N
pinch of flake: 0.5g x 53% protein x 16% N in protein = 42 mg N
Total = input of 40+20+42 = 102mg N in 260L system = 0.39 mg/L of N
if 100% of that protein N went into NH4 that’d give 0.39 x (18 mass NH4 / 14 mass N) = 0.50 ppm NH3+4
Since fish release ~80% of protein input as ammonia, this would take 5 days to reach 2 ppm ammonia. If you feed sparingly, you could probably stretch that out to 2 weeks before total ammonia would go up to 2 ppm, even if your tank consumed zero.
2. Most ammonia is non-toxic NH4. NH3 is a small %. pH really matters. Even if I have the hypothetical 2 ppm total ammonia at pH 8.2, then it’s only 0.13 ppm toxic NH3. see calculator. If the pH drops to 7.8 then 2ppm total ammonia is only 0.05 ppm NH3.
3. Most organisms are tougher than the few sensitive fish we think about. From RHF article
” Marine fish generally have 96 h LC50 levels that range from about 0.09 to 3.35 ppm NH3-N.”
That is, while there are sensitive fish that have a LC50 (lethal concentration to 50% of specimens) over 4 days (96 hr) of ~ 0.1ppm NH3, many saltwater fish are much tougher. So many systems could spend days at the above hypothetical 2ppm total ammonia (pH 8.2, NH3 = 0.13) and not lose livestock. Benthic organisms are even tougher than that, in looking up NH3 tolerances for random inverts in my system, pods, asterinas, snails, crabs, shrimp – all quite high by our sensibilities.
4. We assume new fish will be a little stressed in a new environment “take a few days to settle down.” Many hobbyists might confuse some sub-lethal level of NH3-stress for new to a system stress, and think Prime prevented NH3 toxicity.
5. Heterotrophic bacteria are everywhere (and in some bottled tank starter products). They can reproduce really fast if conditions are right. With addition of carbon – present in all fish food – they can process ammonia quickly also. Plenty fast to avoid bad outcomes. Bottled bacteria myth or fact thread showed repeatedly the headscratching result that adding fish food caused ammonia to go down with these bacteria.
6. There are a zillion photosynthetic organisms that will show up rapidly in any tank that has light. Photosynthetic organism are strong consumers of ammonia themselves, and they release dissolved organic carbon that can help the heterotrophs consume ammonia also.
7. Photosynthetic organisms have such a strong preference for ammonia, that they have a hard time consuming any other form of N, if any ammonia is present. They’ll ignore 20 ppm NO3 to grab 0.2 ppm ammonia. It’s not like they are choosing it, it’s just so much more energetically favorable as a food source.
8 / Putting it all together.
Tank “crashes” / die-offs don’t raise NH3 as much as you might think. A 100g of dead fish that hides in your 55 gal (210 Liter) tank doesn’t immediately turn into a bunch of ammonia. The fish might be ~20% protein, ~16% of protein is N, so 100*.20*.16 = 3.2g N. 3200 mg N / 210 Liters = 15mg/L N = ~19 ppm total ammonia. Protein is broken down gradually over days to a week so maybe only 3 ppm ammonia release per day. Some of that is eaten by clean up crew, that might assimilate 20% into growth and release the other 80% as ammonia. The carbon in the tissues also increases heterotroph activity that reduces how much N is released into ammonia. And on top of that, the metabolic processes involved in breaking down the fish will lower pH as well, thus reducing the fraction of ammonia that is NH3.
If all that decomposing organic material pushes the pH down to say 7.8 (my tank goes that low sometimes without dead fish), then the daily 3 ppm total ammonia release would be only 0.08 ppm NH3. And that’s without even considering the nitrifiers and the photosynthesizers, that with ammonia present will do nothing but eat ammonia, day and night.
So when I lost a couple of small fish to rapid disease and added Prime to “protect” the rest from toxic ammonia, there was never actually a clearly toxic condition to protect them from.
If I wanted to do something I could be sure would help protect fish during an ammonia event:
1) lower pH
2) add algae
3) add carbon (vinegar or whatever carbon you already use in your system)
4) add aeration (heterotrophs and nitrifiers both need O2 to work well, and the die-off could push O2 low.)
5) and y’know… water changes 🙂
Those are all incontrovertible and do not require trusting any unsupported manufacturer claims.
.
Couldn’t do a better analysis if I tried.

Prime and Safe both decompose in water to form quantities of sulfur dioxide gas. Sulfur dioxide gas has a sulfurous sewage smell. Sulfur dioxide gas also can irritate the gills of the fish. In sufficient quantity it can even kill the fish.
This gives the hobbyist three good reasons not to use Prime or Safe:
- No one wants a smelly tank
- No one wants the gills of their fish to be irritated
- No one should want to support a company making false claims
Other False Claims
In addition to the false claim that Prime and Safe detoxify ammonia, nitrite and nitrate, Seachem goes on to make the following false claims about Prime and Safe:
-
Prime and Safe used during cycling won’t affect the cycle time.
-
Prime and Safe are not both sodium dithionite
-
Only Prime and Safe remove chloramine, other conditioners are not effective with chloramines.
-
Prime is 5x more concentrated than competing products
-
Safe cannot be made into Prime. If diluted into a liquid, Safe must be used immediately.
All of these claims are nothing more than snake oil marketing hype with no more validity than the late night commercials for hair loss products or virility enhancing pills. To go to an article proving each claim false simply click on the link on the false claim. All of these claims are proven false by testing delineated in each of the articles.

If you want to compare and look at all the various conditioners go to this link:
5.5.3. Water Conditioners
.
Return to Prime and Safe Menu
Return to Poisons Menu
Return to Conditioner Page
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Dave says
In reply to B. Smith ….. For every true claim about their products Seachem probably makes at least two claims which are simply false. And “false” and “a bit silly” are two completely different things. Take Prime for instance. Seachem claims about seven benefits from Prime. One benefit (Prime neutralizes chlorine and chloramine) is true. ALL and I mean ALL the others are just plain false as proven by testing. I think that goes beyond “a bit silly”.
B.Smith says
I’ve used both prime and safe (more recently bc of the cost savings) for as long as I can remember m, over 15 years I’d say. With many “sensitive” fish and shrimp I have never had a single adverse effect in a tank from it.
It seems to me you guys have a bone going for seachem. I actually use a lot of those products and though some claims or marketing they use is a bit silly, as a whole their shit works.
So what’s your reason for the clear disdain you possess for Seachem?
Dave says
In reply to Steve … Sounds to me like your test kit has “gone bad”. You might check it against a Seachem NH3 test kit. And I have little experience with marine tanks so I can’t comment on the pH.
Steve says
I just stumbled across this as page after finding a stange happenin after using Prime: So i have a reef tank, 2 years old, use a Seneye. Up until recently my nh3 level has always been zero, my nitrates and phosphapes always read zero and PH is 8.2. Recently the NH3 on the Seneye has risen to 0.028ppm and stuck there for weeks, no variation in that reading. I was starting to think my Seneye SUD or slide was faulty so i took out the Seneye and put it in RODI hoping I would see the NH3 drop to zero and prove my Seneye ok. What happened was the NH3 reading increased to 0.035ppm. Was this becuase i put a dirty Seneye in a pot of RODI water and the Amonia increased because of zero bacteria in the water i wondered? So i reached for my bottle of prime, added a high dose in the RODI expecting the Seneye NH3 to immediately drop but the opposite happened, NH3 reading went up to 0.05ppm within minutes! So my Seneye is not “stuck”, i put the SUD back in the tank and the reading has dropped back to 0.03ppm. Now i am very wary of using prime in my tank! This 0.03 ppm is still higher than the regular zero i have been getting. I am currently waiting on some manual test kits to compare the NH3 reading. I have not added or changed anything in the tank so am wondering why i have this stubborn NH3 reading. One thing to note is that at the same time i have seen this NH3 increase the PH has been incredibly static at 8.2, it used to fluctuate from 7.8 to 8.1 during day/night periods but now it has gotten to 8.2 and just sits there 24/7. Could this be why the NH3 reading has gone up and sits at the same 0.03ppm figure 24/7? Should i worry about 0.03ppm? I added some Microbe-lift special blend and nothing happened to the readings.
benz says
kudos for repeating their bogus experiment!!
Dave says
In reply to Greg …. Do a 100% water change on the tank. Put the fish back in the tank. Then do a fish in cycle (cleaning the filter requires redoing the cycle). Don’t feed at all for a week or more. Then feed very lightly for several more weeks.
Greg says
Oh NO!!!! I was hopeful that Seachem Prime was going to save my goldfish. We are just back from vacation and the house sitter was very generous to the fish. We had piles of uneaten food in the aquarium corners and 8 ppm ammonia. Two fish died, the remaining fish are now in plastic buckets while I try to get the cycle under control. I am a novice and Prime sounded so promising online. I cleaned out the filter and did a 50 % water change. Added the bacteria in a bottle stuff to try to jump start the recovery, We are down to about 1 ppm ammonia and 1 ppm nitrite. I added API Ammo-Lock to the buckets. Hopefully that was not wrong. So far they seem to be making it. Is there anything that can speed up the process to get the fish out of the buckets and back in the tank?