
My recommendation with conditioners is simple:
.
Buy ONLY Conditioners that say “Sodium Thiosulfate” on the Bottle in the Ingredients List. Then use 5X the Recommended Dosage.
.
When I used to have chlorinated water for a 60 gallon tank 50% water change I would first off leave the fish in the aquarium. I would drain 30 gallons of water. Then I would take the amount of thiosulfate conditioner which would, per the bottle, condition 30×5 or 150 gallons of water and add it to the 30 gallons that was left in the aquarium. Thiosulfate is just very harmless, even at a 10x concentration. Then I would refill the tank with 30 gallons of water above 70 degrees. Easy.
Cost of Conditioners
There is a huge variation in the effective cost of various conditioners. The most expensive product on the shelves is one hundred times more costly than the least expensive product on the shelves. This is huge.
Calculating the cost of each conditioner for the purposes of doing a 50% water change on a 200 gallon aquarium is as follows:
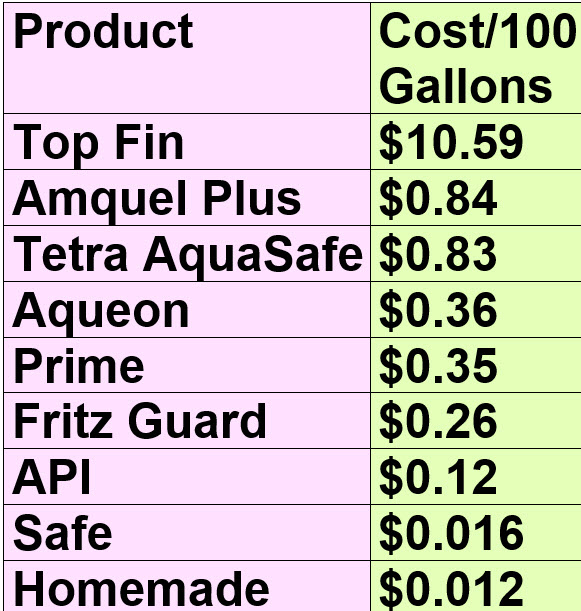

The calculations by product are as follows (calculated for doing a 50% water change to a 200 gallon aquarium):
Top Fin (Thiosulfate) “Use 10 mL for each 10 U.S. gallons”
- (10 ml x 10)/28.3 ml/ounce = 3.53 ounces needed
- 4 ounces cost $11.99
- One-ounce costs $3
- $3 x 3.53 = $10.59 to treat 50% of 200 gallons
Note this product only comes in the small containers and it is an obvious rip off. The author checked these numbers five times and they are valid.
AmQuel Plus (Hydroxymethanesulfinate) “Add 10 drops per gallon, 1 teaspoon per 10 gallons, 1 liquid ounce per 60 gallons, or 1 cup per 480 gallons.”
- (100 x1)/60 = 1.67 ounces needed
- 16 ounces = $7.99 or $0.50 per ounces
- $0.50 x 1.67 = $0.84 to treat 50% of 200 gallons
Tetra AquaSafe (Hydroxymethanesulfinate) “Add two teaspoons for every 10 gallons of water”.
- (10 ml x 10)/28.3 ml/ounce = 3.53 ounces needed
- 8 ounces cost $7.97
- One ounce costs $7.97/33.8 = $0.236 per ounce
- $0.236 x 3.53 = $0.83 to treat 50% of 200 gallons
Aqueon Water Conditioner (Thiosulfate) “Use attached dosage cap and add 5 ml (1 tsp) per 10 gallons of water”.
- (5 ml x 10)/28.3 ml/ounce = 1.77 ounces needed
- 16 ounces cost $3.29
- One-ounce costs $3.29/16 = $0.206
- $0.206 x 1.77 = $0.36 to treat 50% of 200 gallons

Prime (dithionite) “Use 1 capful (5 mL) of Seachem Prime for each 200 L (50 US gallons) of new or replacement water.”
- (5 ml x 2)/28.3 ml/ounce = 0.353 ounces needed
- 500 milliliter costs $17.89
- 500/28.35 = 17.64 ounces costs $17.89
- One-ounce costs $17.89/17.64 =$1.00 per ounce
- $1 x 0.353 = $0.35 to treat 50% of 200 gallons
Fritz Guard (Proprietary) “Use 1 cap (1tsp/5ml) per 50 U.S. Gallons (189 L). $11.82 for 16 ounces”
- (5 ml x2)/28.3 ml/ounce = 0.353 ounces needed
- 16 ounces costs $11.82
- One-ounce costs $11.82/16 = $0.74 per ounce
- $0.74 x 0.353 = $0.26 to treat 50% of 200 gallons
API Tap Water Conditioner (Thiosulfate) “Add 1 ml for each 20 gallons of aquarium water”.
- (1 x 5)/28.3 ml/ounce = 0.177 ounce needed
- 16 ounces cost $11.19
- One-ounce costs $0.70
- $0.70 x 0.177 = $0.12 to treat 50% of 200 gallons

Safe (dithionite) “Use 200 mg of Safe™ (included scoop) per each 200 L (50 US gallons)”
- 200 mg x 2 = 0.400 grams needed for 200-gallon 50% water change
- 250 grams cost $9.99
- One-gram costs $0.04
- $0.04 x 0.4 = $0.016 to treat 50% of 200 gallons
DIY solution of Sodium Thiosulfate is 32 grams of sodium thiosulfate in one cup 8 ounces of water. Add one teaspoon of the solution per 50 gallons of the water to be treated.
- 32/28.3 = 1.13 ounces added to eight ounces.
- 16 ounces sodium thiosulfate costs $3.99
- one-ounce cost $0.25.
- 13 ounces costs $0.28.
- 8 ounces = 48 teaspoons
- $0.28/48 = $0.0058 per teaspoon
- $0.0058 x 2 = $0.012 to treat 50% of 200 gallons
With the notable exception of the Top Fin product, none of these products will exactly break the bank. So most hobbyists don’t worry too much about the cost of their conditioners. If one has a lot of aquariums this will become more important.

Cheapest Conditioner
Note the cheapest conditioner can be made by dissolving 32 grams of sodium thiosulfate in one cup of water (tap water is fine). You don’t want to mix up too much as the solution does go bad with time (like two years time). Add one teaspoon of the solution per 50 gallons of the water to be treated. Since there are 100 drops in a teaspoon, this is a rate of two drops per gallon. To go to the 5x simply go to five teaspoons per fifty gallons, ten drops per gallon.
The metric equivalent is 34 grams sodium thiosulfate in 250 grams of water. Add five milliliters solution per 200 liters of water to be treated (half a drop per liter). To go to 5x simply add 25 milliliters solution per 200 liters of water to be treated.
The math on this is lengthy but pretty easy. This math is designed to neutralize 1 ppm of either chlorine or chloramine as tested by total chlorine test kits.
- Sodium thiosulfate pentahydrate Na2S2O3(5H2O) has a molecular weight of 248.
- Chlorine has a molecular weight of 35.5.
- 248/35.5 = 7.
- Na2S2O3 + 4 Cl2 + 5 H2O -> 2 NaHSO4 + 8 HCl
- One molecule of thiosulfate neutralizes eight atoms of chlorine
- So each 1 ppm of chlorine needs 7/8 ppm or 0.875 ppm of sodium thiosulfate.
- Each 50 gallons will have 189,271 grams of water.
- 1 ppm in 50 gallons is 189,271/1,000,000 = 0.19 grams
- At 1 ppm of chlorine (normally the “maximum” dosage of chlorine in most municipal water supplies) there will be 0.19 grams of chlorine in fifty gallons of water.
- 0.19 x 0.875 = 0.66 grams of sodium thiosulfate required to neutralize 1 ppm chlorine in 50 gallons of tap water.
- So one teaspoon of the conditioner will need 0.66 grams of sodium thiosulfate in it.
- There are 48 teaspoons in a cup.
- 48 x 0.66 = 32 grams
Municipal water suppliers aim for 0.5 ppm in the water. So normally one is safe at these levels of conditioner, neutralizing twice the usual level of chlorine.

But the water can be much higher in chlorine. Municipalities commonly “chlorine pulse” and go to 4 ppm, which requires twice this dosage. And I have personally measured 11 ppm, which smelled like a pool which had just been chlorinated. So I’m not very confident the 4 ppm EPA limit is followed at all times. This is why I recommend using a 5X dosage for thiosulfate conditioners. Because thiosulfate is such a mild reducing agent, a 5X dosage is harmless to fish.
Check with you local water company as to the levels of chlorine and chloramine they might “pulse” with. In some localities it might be wise to test the water with a total chlorine pool test kit before adding the conditioner.
Note that some do the math on various recommended levels from the internet and get all confused as to the exact amount of thiosulfate to use per cup of water. The following recommendations can be gleaned for the web: Jonah’s Aquarium 28 grams, Ken’s Fish 31 grams and Joey Mullen 26 grams. These differences just reflect differences of calculation and formula. It’s not important.
Do not add the crystals of sodium thiosulfate directly to the aquarium. During the time it takes for the thiosulfate crystals to dissolve the fish will be exposed to chlorine. And ANY exposure of the fish to chlorine is to be avoided.

Further Reading
There are some aquarium hobbyists who are interested in delving deep into the science and the calculations behind all aspects of the hobby. For those who are so inclined the following is pertinent:
5.3.3. Water Conditioners
5.5.3.1. “Ammonia Detoxifying” Claims
5.5.3.2. Seachem Prime and Safe
5.5.3.3. Water Conditioner Chemistry
5.5.3.5. Water Conditioner Testing
5.5.3.6. Review Of Water Conditioners
.
Return to Poisons Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Jimmy says
Hi Dave, I would like your help in calculating how much sodium ascorbate to use to treat heavily chlorinated tap water. So far, my calculation says that 6 grams of sodium ascorbate treats 10 gallons of tap water with a chlorine concentration of 10ppm. Could you please help verify that this is correct? Thank you!