
Ammonia, Nitrite, Nitrate, and Chlorine are the four water parameters of interest here. Many people consider them all to be potent poisons of fish. In truth, only chlorine is a potent poison for fish. Ammonia and nitrite are only toxic at some surprisingly high levels of the toxin, dependent on pH. And nitrate is relatively non-toxic.
The many myths about ammonia, nitrite, nitrate, and chlorine are:
- Nitrates going above 10, 20, 40 or even 80 ppm will shorten the life of an adult fish.
- Long term ammonia levels at some level below 5 ppm or nitrite levels below 1 ppm at a pH of 7 decrease the life expectancy of fish because of toxicity.
- Short term ammonia levels at 5 to 10 ppm or nitrite levels at 1 ppm to 5 pm are very toxic to fish at a pH of 7 and will kill the fish rapidly.
- When a lot of fish die overnight after a water change it is typically due to ammonia, not a chlorine pulse, low oxygen or high carbon dioxide.
- The standard dose of conditioner will remove even heavy chlorine or chloramine levels.
- There are chlorine water conditioners such as Prime which “neutralize” ammonia, nitrite, and “toxins”.
- It is important to test your water frequently with test kits such as API Master test Kit, Nutrafin Test Kit, Tetra easy strips, Sera Aquatest, API 5 in 1 Test Strips, or the Fluval Water test Kit.
.
All these myths are simply false.
.

Toxic Levels
Acute toxicity is when the fish die in hours or days. This is the “Toxic” level in the chart below. Chronic toxicity is where the fish will have diseases and a general “failure to thrive”. This is the “Alarm” level below.
The levels are as follows:
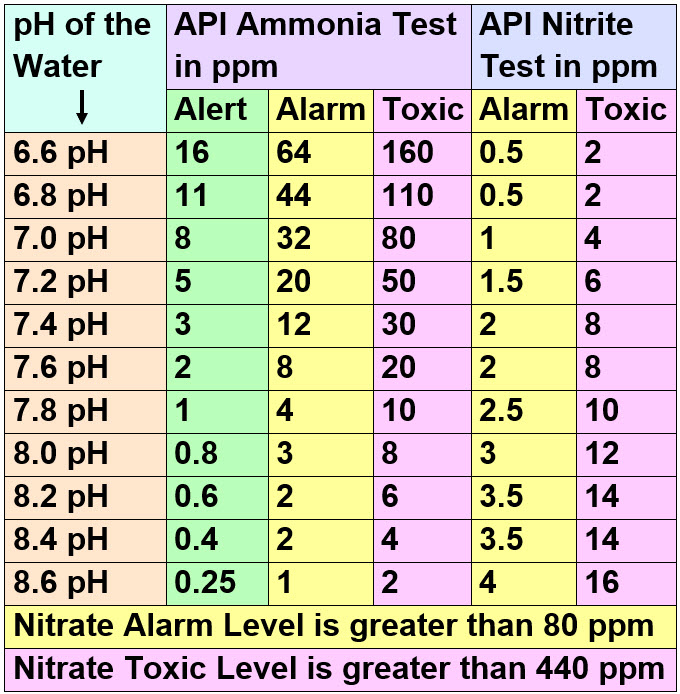
To read the ammonia and the nitrite levels on this chart find the pH of the aquarium first. Then find that pH in the left column. Read across as to the levels the API tests will show. Green is the “Alert” level where increased testing is called for, yellow is the “Alarm” level where one should do a 50% water change. And “Toxic” is the level where one should do a 75% water change. Note many of these levels require diluting the aquarium water with 9 parts of distilled water. Test the diluted water. Multiply the results by ten and you have the levels in the aquarium.
The numbers for the toxicity of ammonia, nitrite, and nitrate will come as a shock to most experienced hobbyists. But the numbers are supported by a lot of research by universities. The ammonia levels are what Seachem delineates. They are solid. Ammonia, nitrite, and nitrate are not as toxic as we have all been led to believe. In general fish from lakes or ponds have a higher tolerance for nitrogen compounds than do fish from rivers.

Chlorine
Chlorine is toxic in very small quantities, on the order of 0.05 ppm chlorine. Chlorine is a very bad actor. The reason for this is simple. Free chlorine gas is not found in nature. Ammonia, nitrite, and nitrate are all found in nature. In the case of a lake with high organic matter, the levels of the three nitrogen compounds can get surprisingly high. So Mother Nature has provided most fish with coping mechanisms for these three naturally occurring nitrogen compounds. Nature has provided no such coping mechanism for chlorine.

Testing the Water Parameters
One constantly hears on social media the question: What are your water parameters? I.e. what are your ammonia, nitrite, and nitrate levels? And this supposedly gives one a clear picture of whatever disease the fish has. This is simply hogwash. There is simply no way to determine what disease a fish has from the water parameters.
A related myth is that it is that somehow it is important to test your water frequently with test kits such as API Master test Kit, Nutrafin Test Kit, Tetra easy strips, Sera Aquatest, API 5 in 1 Test Strips, or the Fluval Water test Kit. This makes a lot of money for a lot of manufacturers of test kits but it does little good for most aquarium hobbyists. People end up obsessing over it.

Ammonia and Nitrite Levels to Strive For
If one starts having sick fish one needs to measure the “water parameters”, namely nitrite and ammonia. The reasoning behind this is NOT because ammonia or nitrite are horribly toxic, they are not. But rather high (>0.25 ppm) nitrite and ammonia indicates the biofiltration on the aquarium is inadequate.
If the biofiltration is inadequate, dissolved organic compounds and bacterial count in the water can get high. High dissolved organics cause high bacterial count in the water. High bacterial counts in the water are one of the biggest killers of fish. So ammonia and nitrite levels become the “canary in the coalmine” for an aquarium.
.
The ammonia and nitrite levels per the API tests should always be 0.25 or lower, not because ammonia or nitrite are that toxic, but rather because any ammonia or nitrite indicates poor biofiltration which will kill fish from excess bacteria in the water.
.

Note all hobbyists should have aquariums with 0 ammonia and 0 nitrite after a few months of operation. The reason behind zero ammonia and zero nitrites has nothing to do with the toxicity of ammonia or nitrite. As mentioned above, it has to do with bacteria count in the water.
It has to be emphasized that achieving zero ammonia and zero nitrite takes time. An aquarium needs to be established for three or four months before this goal can be achieved, even with huge amounts of surface area in the filter. And it must be emphasized that in the meantime short excursions of up to 5 ppm ammonia, 2 ppm nitrite, and 880 ppm of nitrate or even higher for most fish will not harm the fish at a pH of 7.0.

Further Research
If one is more interested in the science and all the research papers behind all this simply read the following links:
5.1. Deleted article
5.2. Safe Ammonia Levels
5.3. Safe Nitrite Levels
5.4. Safe Nitrate Levels
5.5. Chlorine and Chloramine
.
Return to Home Page and Main Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit-making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Frank T says
Hi Dave,
I find myself referencing your material often in various settings for all kinds of reasons.
And then I shuddered at the thought this website goes poof one day.
So, have you ever considered packaging up the info into an e-book, even if just a single pdf? I am sure nerds like me would buy it.
Dave says
In reply to Brad I analyzed that research and found it to be a classic case of assimilatory denitrification followed by some very questionable analysis, I reviewed it in this article. This article also includes an extensive analysis as to why reduction of nitrate to nitrogen gas is impossible in the aquarium.
http://aquariumscience.org/index.php/7-5-denitrifying-media/
More to your question there is not good way to stop the marketers. As long as there are desperate grad students who need a sponsor there will be marketers around to take advantage of them.
Brad - UK says
Hi Dave,
What is your view on the below in regards to Siporax?
https://www.icloud.com/iclouddrive/066QdJg7PNNjgmryG5TKCXnOw#siporax_Uni_Sydney_denitrification
I can’t see any evidence from your testing that this is a great filter media. But when a company, people, or user links to this, how do you even begin to argue when you are a layman?!
Brad
Dave says
In reply to Sammy ….. Conditioner can be made by dissolving 32 grams of sodium thiosulfate in one cup of water (tap water is fine). You don’t want to mix up too much as the solution does go bad with time (like two years time). Add one teaspoon of the solution per 50 gallons of the water to be treated. Since there are 100 drops in a teaspoon, this is a rate of two drops per gallon. A five X dosage is ten drops per gallon or five teaspoons per fifty gallons.
Sammy says
I finally found sodium thiosulfate crystals… surprisingly difficult
The label states to use 0.2 grams per 10 gallons.
How would I go about making a solution to keep in a 500ml bottle for dechlorinating tap water at say 5ml for the 5 gallon pails (1ml per gallon would be easiest to remember) that I use for water changes.
The town water chlorine content varies wildly so the 5x dose would have to be standard.
Dave says
In reply to Steven …. I recommend buying solid sodium thiosulfate and making your own conditioner.
Steven Akins says
What brand of water conditioner do you recommend?
ben z says
@Dave
I just saw your reply. will forward the book to u (its in PDF form). But uh, Gerardi, being one of the more recognised experts in his field, generally doesn’t cite sources very meticulously. The book itself is more of a textbook. but there is a bibliography. Also, the book is about municipal wastewater in general – and the sulfites they use are stronger, so that might be a factor.
and uh, a comment about thiosulfate crystals in general (from very solid personal experience). putting solid thiosulfate directly in the water, like someone mentioned, will kill fish 99% of the time.
But it’s more convenient to store thiosulfate in solid form than in a homemade liquid. So what I do is that I throw the crystals into the empty bucket/tote that I use for water changes, then I turn on the tap. The time it takes to fill the container plus the mixing action from the tap will ensure the crystals dissolve and dechlorinate.
I think it’s also better to dechlorinate the water in a separate container rather than add conditioner to the tank and then hose tap water in. Per virtually everything I’ve read, it takes a stoichiometric excess of any sulfite to reduce the chlorine in tap water because the reactions are slow, and also because chlorine molecules like to react with other stuff before they meet a thiosulfate ion. And per my (very unprofessional) back-of-envelope estimations, I think the chlorination levels these days can be so high that even a 5X dose might not cut it if you do the “conditioner then tap water” method. It will work most of the time – but it only has to fail once. I personally think that one shouldn’t chance it at all with small fish (smaller gills).
Dave says
In reply to Ben Z …. Whoh. I need to test this. If one has nitrates in the water and one uses an overdose of conditioner, by this reference considerable nitrites would be produced, possibly killing fish. I’ve never heard of such a thing happening. HHHmmmm have to think about this. Is there a paper referenced in the book? Thinking about it, dechlorinator cannot reduce nitrate. Nitrate is a very weak oxidizer while conditioners are all weak reducing agents. There would need to be a source of considerable energy for nitrate to be reduced by conditioner. This is NOT to say conditioner might not interfere with the test in some way.
ben z says
I just came across this paragraph in the book Nitrification and Denitrification in the Activated Sludge Process (Gerardi 2002):
“The wastewater sample selected for testing should not be taken after chlorination or dechlorination. Chlorine oxidizes nitrite ions to nitrate ions, while dechlorination reduces nitrate ions to nitrite ions. These samples do not provide for a proper measurement of the amounts of nitrite ions and nitrate ions that were produced bio- logically in the aeration tank. Chemicals commonly used for dechlo- rination are sulfur dioxide (SO2) and sodium bisulfite (NaHSO3).”
if this is true, it seems like a very good argument for dechlorinating water in a bucket before adding it to the tank, especially if one has smaller fish. (Bigger fish, logically, would be less affected by any temporary toxicity).
bz says
sodium thiosulfate reaction with chlorine produces “rotten egg” smell?
https://awwa.onlinelibrary.wiley.com/doi/full/10.1002/j.1551-8833.2003.tb10319.x
assuming that odorous compounds are present in some aquariums, that might explain some temporary, weird smells during water changes.