
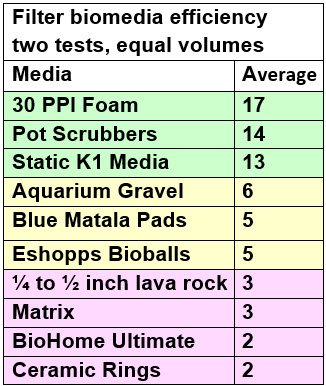
A test of the ammonia oxidizing capability of ten different filter media was undertaken. Ceramic rings, lava rock and BioHome were the worst media. Bioballs and Matrix were twice as good as the rings. Aquarium gravel and Matala pads were three times as efficient as the rings. Static K1 media and pot scrubbers were five time more efficient than the rings. And 30 ppi Poret foam (“sponge”) was nine times more efficient than the rings.
Note that the foam used was very carefully cut so that there were no gaps around the foam. Foam only works if the water flow is forced to go through the foam. If the water flow can bypass the foam it will. This is why chunks of foam are a poor biomedia.
These results were compared to the mathematically calculated surface area of the media and the results were closely correlated, confirming that the effective surface area is the determining factor in the efficiency of aquarium biomedia. This close correlation validates the test very well. Powerful JMP statistical software (which handles multiple variables and no replications with ease) said the three colors of the groups were indeed different at the 95% confidence level.
In addition four media were tested in forty gallon aquariums with canister filters. The results very closely correlated with the results in the other two tests. This again validates the testing.
These were easy tests easily replicated by any hobbyists interested in accuracy. This was very deliberate. We deliberately avoided the type of test which might be “scientifically correct” but difficult to reproduce at home. We encourage anyone to duplicate these tests.
What follows is a very lengthy, verbose and boring dissertation on the testing. It will only be of interest to real aquarium nerds like the author.

Ammonia Oxidation Test Results
Ammonia was added to tanks with filters with various filter media. The resulting ammonia levels were measured three days later and the amount of ammonia added adjusted according to the measured ammonia level. The pH was kept above 7.0 by adding baking soda when the pH dropped. These are the results reported as the number of milliliters ammonia solution added on the day noted:
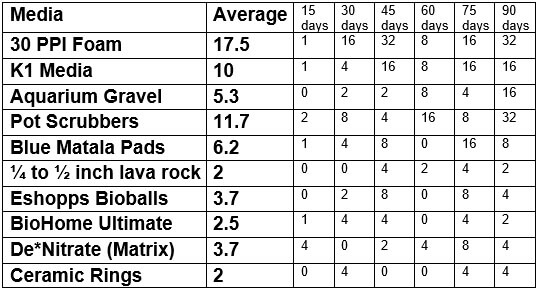
.
Replication #1
The test was then replicated with new media. These were the results:
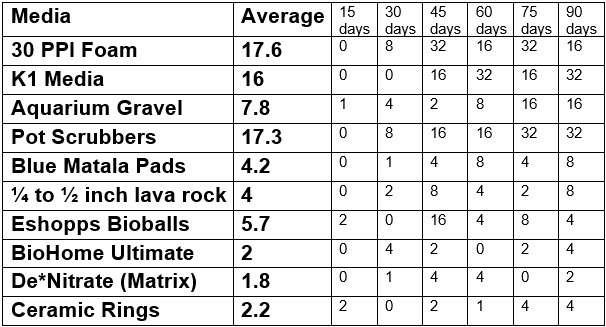
The data replicated reasonably well. Examination of the the pot scrubbers showed they had been stuffed in more densely than the first test, explaining the better showing.

All the data was then analyzed with the JMP software. This software is very powerful, does not require ANY replicates and can handle multiple variables. The group of 30 ppi foam, plastic pot scrubber and K1 was significantly different from the group consisting of aquarium gravel, Matala pads and bioballs (p<0.05). In turn the group consisting of aquarium gravel, Matala pads and bioballs was significantly different from the group consisting of lava rock, Matrix, BioHome and ceramic rings (p<0.05). The group of 30 ppi foam, plastic pot scrubber and K1 was significantly different from the group consisting of lava rock, Matrix, BioHome and ceramic rings (p<0.01).
The average of the two tests is the colored chart above in the Abstract. The exact procedure used is delineated below under “Bucket Test Procedure”.

Test #2 Ammonia Oxidation with Canisters
There is a valid criticism of the above tests that is along the lines of “an air operated corner filter is not a real world test”. So we tested the media performance of two “good” media versus two “bad media” in actual aquariums with canister filters. The data confirmed the validity of the first tests with the corner filters.
Four forty gallon tanks were set up with Sunsun HW 304 B canister filters (~one half cubic foot of media, 370 GPH) on them. Various media was then placed in the filter. The media used were foam, plastic pot scrubbers, Matrix and Biohome. Each canister filter was filled 100% with the media selected.

Each of the tanks was well aerated by a wavemaker aimed at the surface to produce a large area of “choppy waves”. Brown squeezings from an established tank and sodium phosphate were put in at the start of each test.
Concentrated ammonium sulfate was added to tanks with various filter media. The resulting ammonia levels were measured three days later and the amount of ammonia added adjusted according to the measured ammonia level.
The pH was kept above 7.4 by adding baking soda when the pH dropped. The first two “bucket” tests above doubled the amount of ammonia if there was no ammonia. This test with canisters added ammonia in more gradual increases or decreases, giving a decidedly more predictable a curve.

These are the results reported as the number of milliliters of ammonia solution added on the day noted:
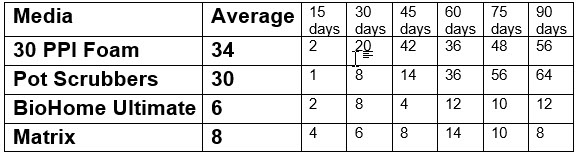
These results confirmed the validity of the first two tests very well with real aquariums and real canister filters.
Discussion of Results
These tests are easily performed by anyone who wishes to duplicate it. It appears the surface area calculations for the pot scrubbers listed elsewhere was way off. There also seems to be a decided bias which makes media which has a great deal of porosity much better than a solid media. This would explain the poor showing of the aquarium gravel. The poor showing of the lava rock was unexpected.
- Note that foam (“sponge”) is a great biological filtration media and does NOT function as a mechanical filtration media as long as it is less than 30 ppi.
- Note that the flow of water must be 100% through the foam. There can be no gaps. And chunks of foam are a very poor media.
- Also note that filter floss (polyester fiber, “Polyfil”) wasn’t tested. Packed filter floss fills with debris and plugs in a week or so and needs to be cleaned or replaced. So packed filter floss can’t build up any beneficial bacteria and functions only as a mechanical filter.

.
Bucket Test Procedure
This is the procedure used for the first test. To test the ammonia oxidizing capability of various aquarium filter media 10 five gallon buckets were set up. The filter media was put in ten air operated corner filters.
Brown water squeezed from well established sponge filters was added to each bucket initially to “seed” the cycles. To create the inoculate solution, a bucket was filled about halfway with water. Six long-established sponge filters from some breeding tanks were squeezed several times in the one bucket. A cup (8 OZ.) of this brown water was then added to each of the ten five-gallon buckets.
Each of the ten buckets was filled to the brim with 8.1 pH well water. The level was only topped off when it dropped. The media was not cleaned during the test nor was the water changed. The pH was kept above 7.4 by adding baking soda when the pH dropped. Nitrates and nitrites were not measured.
Each bucket had four milliliters of ammonia added every day for the first eight days to start the cycle. Each bucket also had one quarter teaspoon monocalcium phosphate added initially to provide phosphate for the beneficial bacteria.

After nine days, if the ammonia test was at or below 0.5 ppm. the number of milliliters added was doubled. If the ammonia test was between 0.5 ppm and 4.0 ppm. the number of milliliters was kept the same. Above 4.0 ppm and above of ammonia, the number of milliliters was cut in half, with half of one drop being zero drops. The ammonia level was measured every three days. Ammonium/ammonia nutrient solution was added accordingly daily. The test was run for 90 days.
Also the additions are the number of milliliters of the solution added each day, not ppm.
Test Equipment:
- 10 five-gallon Home Depot buckets
- Well water (pH of 8.1)
- 60 grams BASF ammonium chloride dissolved in 1 liter water
- 10 Lee’s Economy Corner Filters

Math
• A milliliter of solution is 1 gram of water
• 60 grams ammonium chloride per 1 liter water is a 5.7% solution of ammonium chloride (60/(1000 = 60)) = 5.7%
• 5.7% solution of ammonium chloride is a 2% solution of ammonium (N=15, H=1, Cl=35 …. ((1×4) +15/(35 + 19) x 0.057) = 0.02
• 0.02 grams ammonium per gram of solution
• At a feeding percentage of 1.5% of the weight a pound of fish eats 6.81 grams of 43% protein food per day. Protein is 17% ammonium.
• A “metabolic pound” of fish thus puts out 0.5 grams ammonium per day fed at 1.5% (6.81 x 43% x 17% = 0.5)
• A “metabolic gram” of fish thus puts out 0.00110 grams ammonium per day fed at 1.5% per day (0.5/454 = 0.00110)
• So each milliliter of the above solution is 0.02 grams ammonium.
• One milliliter is then equivalent to 18.18 grams of fish “metabolic weight” (0.02/0.00110 = 18.18) fed at 1.5% per day
• Lee’s economy filters have only about 15 cubic inches of media
• There is 1728 cubic inches in a cubic foot (12x12x12 = 1728)
• Lee’s economy filters have only about 0.0087 cubic feet of media (15/1728 = 0.0087)
• So the solution is being filtered out by 0.0087 cubic feet of media
• So each milliliter of the above solution is 0.02 grams ammonium.
• So the ceramic rings have 2 milliliter being filtered out by 0.0087 square feet.
• So each 2 x 0.02 grams of ammonia is being filtered out by 0.0087 cubic feet of filter with ceramic rings.
• 2 x 0.02 grams /0.0087 = 4.6 grams ammonia per one cubic foot of ceramic ring media
• A “metabolic gram” of fish thus puts out 0.00110 grams ammonium per day fed at 1.5% per day (0.5/454 = 0.00110)
• 4.6 grams ammonia per cubic foot/.00110 grams ammonia = 1.05/.00110 = 4,182 grams per cubic foot
• 4,182/454 = 9.2 pounds of fish per cubic foot of media
• If each cubic foot of ceramic media has 40 square feet of surface area that is 9.2/40 = 0.23 pounds of fish per square foot or 1.15 pounds per five square foot. This is very close to the five square foot per pound in the literature.

Test Interpretation
This simple test can be reported in several different formats:
In the format below the first number is the average ammonia oxidizing that roughly 15 cubic inches of media accomplished over a 90-day period. The second number is the effective surface area in feet squared per feet cubed, calculated by simple math. The correlation between the test results and the calculated surface area is very significant and means both are good ways to judge the efficacy of filter media.
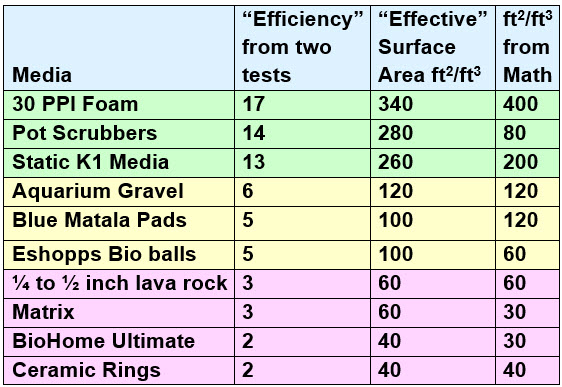
It appears the surface area calculations for the pot scrubbers was way off. But other than that the numbers correlate quite well.

In Depth Discussion
Because of reticulation, flow and free volume considerations the surface area calculations are not very dependable. Extrapolating from the testing and giving aquarium gravel the most dependable surface area calculation, gives the following “effective surface area” by media. This is the surface area which should be used for calculating the required volume of filter media for a given weight of fish.
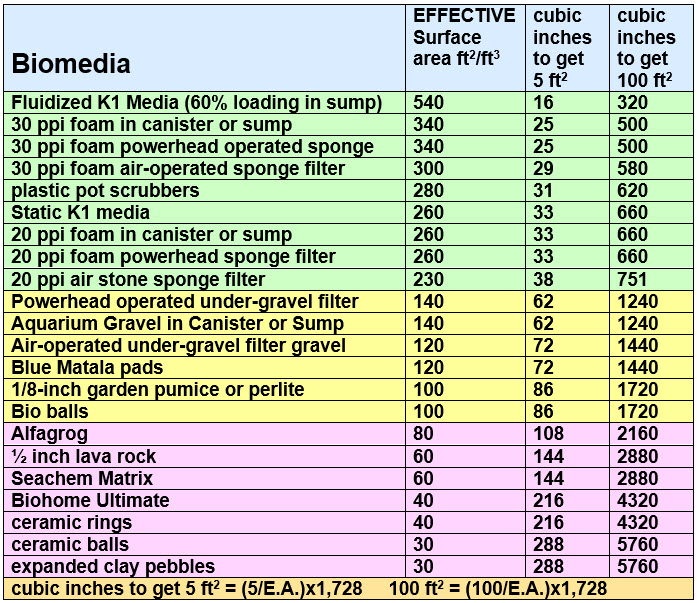
.

.
Data Interpretation
To give one an idea of what this test data means, if a SunSun HW 303 canister is filled with each of these media, it would support this number of ten inch Oscars or four inch mbuna for ammonia oxidation:
- 30 ppi foam 36 Oscars or 720 mbuna
- Pot scrubbers 24 Oscars or 480 mbuna
- K1 Media 20 Oscars or 400 mbuna
- Matrix 8 Oscars or 160 mbuna
- Lava Rock 4 Oscars or 80 mbuna
- Ceramic Rings 4 Oscars or 80 mbuna
These are startling numbers most won’t believe. But this is for only ammonia oxidation. Ammonia oxidation is EASY! The next test determined that for crystal-clear water each of these numbers needs to be divided by twenty.
These are the numbers for crystal clear, healthy water from a SunSun Hw303 canister filter:
- 30 ppi foam 2 Oscars or 36 mbuna
- Pot scrubbers 1 Oscars or 24 mbuna
- K1 Media 1 Oscars or 20 mbuna
- Matrix 0 Oscars or 8 mbuna
- Lava Rock 0 Oscars or 8 mbuna
- Ceramic Rings 0 Oscars or 4 mbuna
This explains why it is so difficult for many hobbyists to get crystal clear water.

This is a very small amount of surface area required for ammonia oxidation and is the reason that even small cartridge filters with poor media can do decent ammonia oxidation if the cartridges are not replaced once a month. This comes down to a simple guideline:
.
Ammonia oxidation is easy and does not require a top end filter media.
.
Cory at Aquarium Co-op has an excellent video (presented courtesy of Ben Ochart) where he talks about how little surface area is actually needed to do ammonia oxidation. Cory said he had only seen ammonia spikes and readings in newly set up aquariums. He had never seen them in aquariums that had been established for several months, irregardless of the filter media or the filter. But Cory misses the fact that much more filter media surface area is required for a healthy, disease free aquarium than is required for an ammonia free aquarium.

Thinking about it I would have to echo what Cory said about ammonia. Regardless of the filter or the filter media I’ve never seen ammonia in any aquarium over three months old. And I’ve had many many tanks for some fifty years.
What I have had in many aquariums is dull semi-cloudy water. This dull semi-cloudy water is always accompanied by lots of disease: fish TB, hole-in-the-head, septicemia, epistylis, tetrahymena, etc. All these problems did not occur in aquariums with “over-filtration” with the better filter media. The reason has to do with the bacterial count in the water. If one has ten to twenty times more filter media effective surface area than ammonia oxidation requires, one will have clear bacteria free water with healthy fish. This is the point Cory missed.

.
Support from University Research
This data point of five square feet of surface area per pound of fish being required to oxidize ammonia is a figure which is confirmed by no less than four aquaculture studies (“Constructing a Simple and Inexpensive Recirculating Aquaculture System (RAS) for Classroom Use”, David Cline, Southern Research Aquaculture Center, Auburn University, 2005, “Aquarium Culture of Tilapia”, James E. Rakocy, University of the Virgin Islands, 2005, “Production in Intensive and Recycle Systems”, Muir et. al., 2009, “Nitrification in Moving Bed and Fixed Bed Biofilters Treating Effluent Water from a Large Commercial Outdoor Rainbow Trout RAS”, Suhr, 2010). The number is quite firm.

An Anecdotal Piece of Evidence
I generally don’t like anecdotal evidence but this one was interesting. One Jim O’Neill, on the Facebook forum Ben O’Cichlid, did a test of foam as a media and got great results:
“I know that the web site Aquariumscience.org incites controversy here, but I have, over the past week, put some of this websites advice to the test with good results.
I set up a new 20-gallon tank with a new Fluval 207 filter. I seeded the filter with two of the vertical prefilter foams from an operating Fluval 206 and with the Fluval Bio-Foam, which had been in the bottom tray of the 206. I left the new blue bumpy foams in place in the new 207 and I put two more Bio-Foam sponges in the other two trays. So the filter is filled with entirely foam. No other media of any sort.
I also squeezed gunk from a few other sponges in existing filters into the water.
The tank was instantly cycled. I put nearly a full stock of fish in it (ready to remove them instantly if any ammonia or nitrite appeared. I have tested the tank twice a day for six days now, without a trace of ammonia or nitrite. I’ve used both API and Tetra strips (for nitrite only–I don’t have the ammonia test strips), the API liquid tests, and the Seachem nitrate/nitrite test.
So I can’t confirm that foam is better than any other media for biological filtration, or that it can support a higher fish load, but I can confirm that, under the circumstances I’ve described, it does the job for a normal load and that there is no need for expensive biomedia.”
Interesting test.

Aquaculture Tests of Stationary Filter Media
For many years there were a host of tests done on stationary filter media by the aquaculture industry. For example a test would test large gravel versus small gravel versus bioballs versus ceramic rings versus vinyl noodles in a large number of 5,000 gallon tanks where Tilapia were being raised for human consumption.
The test invariably showed very random results which were “all over the map” in common parlance, “not statistically significant” in scientific parlance. Since scientists do not write reports on data which is not significant and proves nothing there are no reports on it. But the fact has been verified by several scientists in the aquaculture industry.
Occasionally some aquaculture student will come on social media and say something along the lines of “When I was taking my aquaculture school course we tested media and found no great difference in media“. This is confusing to some hobbyists. What is being missed here is the loading factor.

All the various aquarium fish stocking websites have a “maximum recommended stocking” of about 1/3 pound of fish per 100 gallons of water. Tilapia farmers stock at fifty (50!) pounds of fish per 100 gallons. So the aquaculture research on filter media was a loading one hundred and fifty times heavier than you will find in most aquariums.
At that high loading there are two competing factors in play. Fouling of the media, slowing down flow, occurs very rapidly. This fouling competes with the surface area. A small media will have more surface area but will foul faster. So the data ends up all over the map. This simply won’t happen in a typical tropical aquarium.

.
Nitrate Removal
There are two media which claim to reduce nitrate to nitrogen gas. This is Biohome and Matrix (actually a form of Matrix called “De*Nitrate”). Testing showed these material did not do any reduction of nitrate to nitrogen gas. This testing can be found in this link:
7.5. Test of Denitrifying Media
.
Fake Science
There is one “fake science” study that is along the same lines as the study above which should be mentioned. This is a fine example of the hubris some manufacturers have. The study is “Comparison of MarinePure™ Bio-Media versus Other Types of Bio-Media on Ammonia and Nitrite Removal in Freshwater Aquarium Systems”, done by the Cermedia MarinePure Lab, October 2, 2012. This is hilarious reading for any scientist familiar with aquaculture filtration systems.
The following is the graph of the “results”.
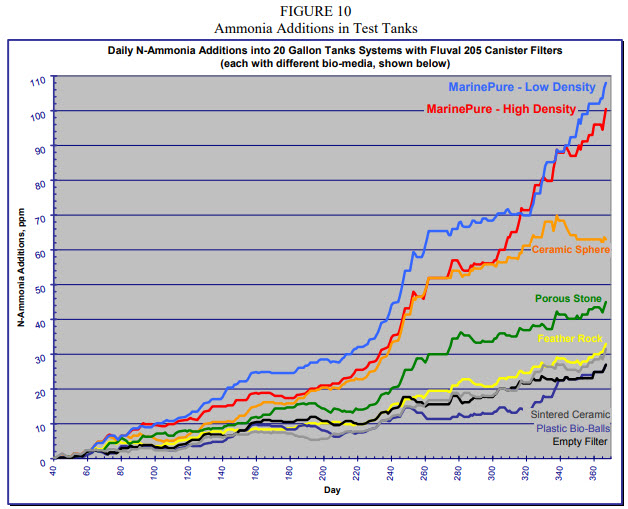
This is obviously faked data. It says that media never plug up and that the media can ALL increase their capacity for ammonia oxidation forever. Which is just ridiculous. Furthermore the lack of “noise” in this study indicates the data is fake. Natural systems simply don’t function this way. Natural systems normally bounce up and down very unpredictably.
They used the same inaccurate API test kits that were used in the study above. Such test kits will give much greater variations based just on their inherent inaccuracy. And how does one make the tiny adjustments to the ammonia level shown in this test with the large numbers of the API ammonia test?
Note that the data starts at day 40 with all the test results indicating ZERO ammonia additions. How does one cycle media with zero ammonia additions? Indeed, at 60 days there is still only less than 2 ppm of ammonia being added. Huh? Since when does any media take that long to even begin cycling?
And an empty filter performed better than a filter with plastic bioballs? And feather rock and ceramic media were about the same as an empty filter? And pigs fly.

Another glaring error is that, per the referenced report: “About once a month the filters were opened and excess bio-film was squeezed off the mechanical sponge filters. The bio-media, still in the media trays were dunked several times in used tank water.” Common sense says that once a month all the media (and especially the easily cleaned bioballs) should have seen a sizable dip in their ability to oxidize ammonia, with all media showing some dip in unison. There is no cyclic dip at all anywhere in the data for any of the media, let alone cyclic dips in unison.
The other point that is indicative of fakery is that the data is all actually pretty much what one would term “straight line” functions. Natural systems are very rarely straight line functions. Since no “seed” inoculate was used and since beneficial bacteria reproduce by splitting in two every 24 to 120 hours, initially each of these curves should have doubled the input allowed every time period (one to five days), resulting in what is called an “exponential curve”.

Then, depending on the media, the curves should have leveled out at somewhere between one and three months. After that the curves should have just bumped up and down at the same level or slightly increasing levels once a month with cleaning. These are what the curves should have looked like (without the up and down “noise”).
.
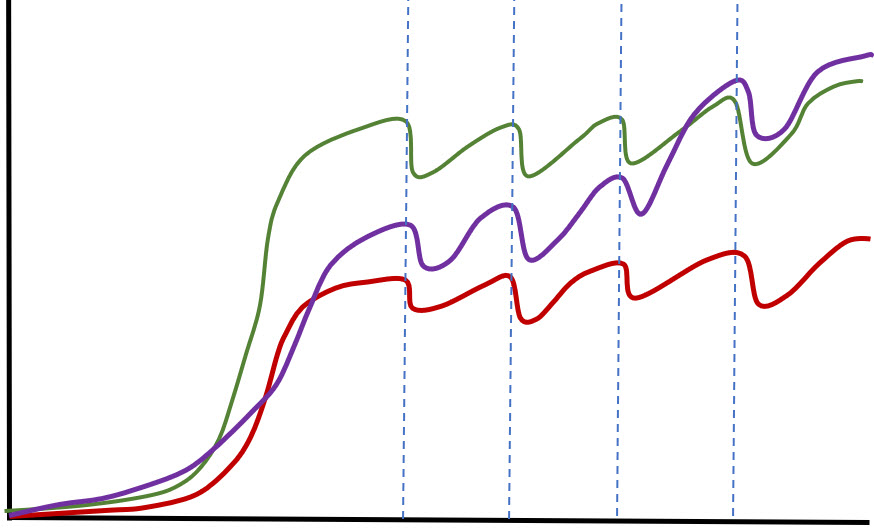
These type curves are obviously is not what this fictitious data follows.
This study concludes that one liter of Cermedia Marinepure low density media can biofilter the ammonia from 21.7 pounds of fish after one year of operation, based on the “actual test results”. When pigs fly. Even the best sand filters used by large commercial aquarium operations can’t hit a number anywhere near this high.
But some will believe this claim. For those people here’s an even better deal: I sell a white pill which is guaranteed to raise I.Q. by 40 points, make one irresistible to the opposite sex, and takes 20 years off your apparent age. It is only $998 per tablet. Just ignore the letters B-A-Y-E-R on the tablets. Honest! Would I lie?

The Ceramic Media Surface Area Scam
Many ceramic media manufacturers (such as Matrix or Biohome) measure the surface area as the area that incredibly tiny nitrogen gas molecules can access. This is a pure scam. The areas they measure are literally tens of thousands of times larger than the actual effective surface area. This scam is explained at length in this link
7.2.11. Ceramic Filter Media
The openings in the media need to be a sizable diameter for bacteria to grow in them. A bacteria is billions of times larger than a nitrogen molecule. If the bacteria can’t fit in a pore with room to spare, the pore is useless. But the huge surface area of these billions of tiny pores IS measured by the nitrogen method. So the huge surface area numbers coming from the ceramic media manufacturers is simply misleading marketing hype.
The small pore size also creates problems even for large pores like 2 millimeter (0.040 inches). The water flows through the path of least resistance. The water will not flow through small 2 millimeter pores if the water can flow around the media. If water doesn’t circulate through the holes of the media then obviously the water can’t have its ammonia oxidized by bacteria.

So the effective surface area of all media becomes the surface area over which water flows, not the surface area that incredibly tiny nitrogen gas molecules can somehow find their way to. This “effective surface area” is what was calculated above.
The higher the number the better the media. So, foam is the best media and ceramic rings are the worst media both by this test and by math calculations. Since foam must be exactly cut to the proper size to prevent flow around, static K1 media or pot scrubbers are much easier to use. But many have great success with foam.
.
Return to Filter Media Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Dave says
In reply to Johnny …. Easy to tell if it is enough. Is the water CRYSTAL clear down the three foot length? If it is CRYSTAL clear you have enough filtration. Otherwise you need to add filtration.
Johnny says
Hi Dave. Do you think 3 inches of gravel (actually 2mm size coarse sand) in 3ft lengthy and 1ft wide tank with under gravel filter running since 5 months is enough for 4 full grown oscars? Also I am planning to make gravel layer from 3 to 4 inches. Thanks.