
Removing Nitrates with Assimilatory Denitrification
Bacteria, fungi, water molds, wood, algae, mulm, protozoa, plants and, indirectly, fish can absorb nitrates, nitrites and ammonia from the water and produce proteins. This can reduce the nitrogen compounds by something called “assimilatory denitrification”. It is common and well documented.
It is relatively common for an aquarium to have zero nitrates if it has plants and few fish. But note that it is not a given. Two apparently equal planted tanks can have one rapidly buildup nitrates while one doesn’t build up nitrates. It is quite unpredictable.
The most successful and easiest way to use assimilatory denitrification is to just use plants to absorb the nitrates (Mr. Hau’s aquariums and Dr. Novak’s Aquariums). We won’t cover that method here. Instead we will limit this article to assimilatory denitrification via “brown gunk” absorption.
Note this is one of many very boring and long dissertations by the author. Read on only if you are a real nerd like the author.

Garbage Can Denitrification
Let us assume one has a level of 160 ppm nitrates in the tap water and one wants to use the water for a shrimp tank. Anecdotally a case can be made that shrimp do not like high nitrates. So how does one reduce the nitrates in the water using assimilatory denitrification? One CAN use “garbage can denitrification” to remove the nitrate.
To do “garbage can denitrification” one can simply set up a large garbage can full of the tap water with a filter on it like a large canister filter. Use foam, K1 or pot scrubbers as the media in the canister. Cycle the garbage can filled with the high nitrate water by any of the many cycling methods.
Then simply add sugar over a period of days till the nitrate drops to an acceptable level. Wait one week then use that low nitrate water for water changes. After taking out the water change water, simply add more nitrate rich water to the garbage can without doing anything to the filter and add more sugar.

Wait at least a week till the sugar is gone from the water and then use the water for another water change in the aquarium. Just keep doing this until the canister slows down in the flow. Then clean half of the media in the canister. When the flow slows again simply clean the other half of the media.
This is a way to use assimilatory denitrification to reduce nitrates. But it is tricky, to say the least. One can only use the water if sugar hasn’t been added for a week or two.

Aquaponic Filtration
Note that this “garbage can” (a shallower large bin is even better) can easily be set up with a heavy duty LED grow light(s) over it. Put an assortment of emergent plants like frogbit, water lettuce, water hyacinth, salvinia, azolla and duckweed in the water. Let them grow and simply remove handfuls of vegetation every once in a while.
Typically one of the plants will take over and grow very rapidly, absorbing nitrates. This “hydroponic” setup simply takes advantage of another type of assimilatory denitrification. Both sugar and plants can be used together.
More about using plants for nitrate removal can be found at this link:
6.6. Aquaponic Filtration

Assimilatory Denitrification in Depth
It is possible to accumulate nitrate in a nitrogen rich proteinaceous “brown gunk” in a filter and then remove the nitrate from the aquarium by changing out the media or cleaning the media. There are a whole series of designs which can do this. But the shortcomings of this method far outweigh the benefits. Chief among the shortcomings is that it must be very carefully controlled to prevent disease breakouts in the fish.
Per the paper “Denitrification in Recirculating Systems: Theory and Applications” van Rijn et. al 2005:
“Organisms capable of assimilatory nitrate reduction use nitrate, rather than ammonia, as a biosynthetic nitrogen source. Organisms capable of assimilatory nitrate reduction include plants, algae, bacteria and fungi. Assimilatory nitrate reduction takes place under aerobic as well as anaerobic conditions. No net removal of inorganic nitrogen is accomplished by this process, since inorganic nitrogen is converted to organic nitrogen“

A huge variety of organisms incorporate nitrogen compounds into their bodies as proteins as they grow. And the pathways that can accomplish this are many and VERY complex. For instance a high carbohydrate content in the food can feed a bacterial slime build up in the filter. This bacteria slime will incorporate nitrates into the bodies of the bacteria as proteins. And frequent cleaning of the filter can then remove the nitrogen in the bacterial bodies.
Another pathway is that algae can grow by absorbing nitrates from the water. Grazing microscopic worms and organisms can eat the algae. Then grazing fish eat the organisms or the algae. And the fish grow by incorporating the nitrogen compounds into protein in the muscle of their bodies. And the aquarist comes on social media and wonder why they never get any nitrates in their aquariums even though they do no water changes,

Carbon to Nitrogen Ratio
There is an interesting relationship which occurs in aquariums. The amount of brown gunk, beneficial bacteria and nitrate produced in a filter media is dependent on the ratio of carbon to nitrogen in the food. With higher carbon to nitrogen ratios (i.e. low protein food) there is less beneficial bacteria produced, more nitrogen containing brown gunk produced (more “assimilatory denitrification”) and less nitrate in the water.
.
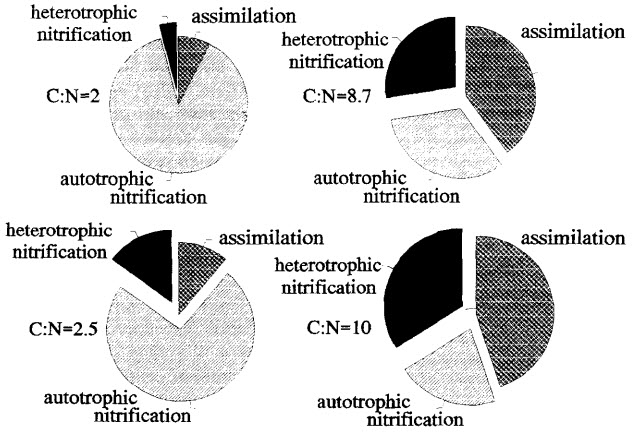
.
It is thus desirable with assimilatory denitrification to utilize a low protein food (a high carbon to nitrogen ratio food). Nitrate is easily tied up in brown gunk in a sponge filter media if the food fed has a high amount of carbohydrates in it. This absorption of nitrate via bio-accumulation is the “assimilatory denitrification” mentioned above. One can also use any carbohydrate as a carbohydrate feed which gets the carbon to nitrogen ratio up high enough to get large amounts of assimilatory denitrification. These carbohydrates include glycerin, sugar and vinegar.

Problems with Assimilatory Denitrification
Using high carbohydrate dosing to produce nitrate reduction by assimilatory denitrification has the following problems:
- It encourages a high dissolved organic compound (DOCs) level in the aquarium water
- Bacteria in the water column feed on the dissolved organic compounds and proliferate
- The high bacterial count requires the fish devote significant portions of its immune system to fighting off this bacteria
- Because the immune system is fish is fighting off the bacteria the fish have fewer resources to fight off diseases
- And the fish get diseased
- The brown gunk surface has few or no “beneficial bacteria” (technically these are “nitrifying bacteria”) so ammonia spikes are common
- Because of the water column bacteria the water will typically not be crystal clear
- The aquarium will often smell bad
- One needs to clean out the filter media often, which can result in more ammonia spikes and is a pain in the butt.
But it is POSSIBLE to utilize a low protein food, cellulose or carbohydrate additions (sugar, vinegar, glycerin) to remove nitrates from the water. One CAN use this process if one is absolutely dead set against water changes or has VERY high nitrate water. The author does not recommend it. Water change or plants are much better solutions. And remember, nitrate is simply not very toxic. Research says most adult fish are fine with no effects from even 440 ppm of nitrate.

Carbon Dosing
This process is used in a somewhat unique method where vinegar, sugar, alcohol, gluteraldehyde or other carbohydrate is added to a saltwater aquarium in what is called “carbon dosing”. The “carbon dosing” creates bacteria in the water column. The bacteria incorporate both the carbohydrates and the nitrates in the water into their bodies. Then the bacteria in the water column are removed by protein skimmers. This can prevent nitrate buildup in saltwater. Unfortunately protein skimmers don’t work in freshwater so it doesn’t work in a freshwater aquarium.

Starch Media and Reactors
There are a series of products (Tetra Balance Balls, AquaMaxx Biopellets, ecoBAK PLUS, NPX BioPlastics, BRS Polyhydroxyalkanoate Biopellets) which are marketed to reduce nitrates which are balls of starch or other polymeric carbohydrates, sometimes designed to be used in special “reactors” and sometimes designed to be just added to a filter.
ALL these products say they reduce nitrates to nitrogen gas by anaerobic denitrification. This is just marketing hype. They ALL are just ways to induce assimilatory denitrification. And they have all the shortcoming of assimilatory denitrification. They work in saltwater with protein skimmers, but are very tricky. They do not work in freshwater tanks.

Cellulose Reactor
In the 1980’s a type of farm reactor was developed where a couple tons of wood chips were buried with provisions for feeding water contaminated with nitrate fertilizers to the wood chips. “Testing” showed a sizable reduction in the nitrate level of the water coming out of the reactor and the device became all the rage of conservationists.
Then someone thought to put radioactive nitrate into the system and measure the amount of radioactivity in the gas coming out of the reactor. They found out there was little radioactive nitrogen gas in the reactor out-gassing. So the nitrate was not being reduced to nitrogen gas. Then they did isotope studies (the ratio of N15 to N14 changes with denitrification of nitrate) and found there was little or no denitrification to nitrogen gas taking place.

What was happening was that bacteria, water molds and fungi were feeding on the cellulose in the wood and taking up nitrate and incorporating it into their biomass as protein. When the cellulose ran out in a few years all the nitrate accumulated was released back into the environment.
The surprising thing is that this was “invented” by a farmer as a way to prevent contamination of the environment. Any gardener will tell you that if you incorporate sawdust into the soil around a plant you will have to add a ton of nitrogen fertilizer because the sawdust will absorb the nitrogen.
Now there is some limited denitrification to nitrogen gas in such a reactor. But it is a hit or miss proposition. Some wood chip reactors will produce considerable nitrogen gas from nitrates while others will not. Conditions have to be perfect, with regions of anoxic conditions being produced in a reactor in which there has to be a good flow of well oxygenated water. This is not an easy thing to obtain.

Aquarium Cellulose Reactors
Some hobbyists have incorporated cellulose reactors into their aquariums and reported huge success with removing nitrates. This will work well if the aquarium is well aerated. When the hobbyist removes the slimy, bacteria laden decomposed cellulose they will be removing a sizable amount of nitrogen from the aquarium. It does take about three months for a reactor to begin working well and absorbing nitrate.
To build such a reactor make up an even number of mesh bags. Fill them with a mixture of 4/5 pea gravel or aquarium gravel and 1/5 wood shavings, wood chips or wood toothpicks. It is important that the gravel media have large openings that allow lots of flow through the cellulose. One cannot use sawdust as the sawdust will clog up the flow in short order.

Put them in a canister or in a sump where there is a flow of well oxygenated water. Replace 50% of the cellulose filled bags on a six to twelve-month schedule. By replacing only 50% of the bags on an alternating schedule one will never be without a mature reactor.
Since this is NOT an anaerobic denitrifying reactor one does not need to worry about flow. Simply flow a goodly amount of well oxygenated water through cellulose of some sort (wood shavings, large wood chips or wood toothpicks) and let Mother Nature accumulate the proteins for you to clean out occasionally.
The biggest problem is that the cellulose (or any carbohydrate) produces something called “dissolved organic compounds” or “DOCs”. These DOCs feed bacteria in the water column. Not only does this produce cloudy water but it is very unhealthy for the fish. This is the chief reason I do not recommend assimilatory denitrification.

Another common problem with such a “reactor” is smell. It is extremely important to aerate the water very well before it gets to the cellulose. This will normally prevent a bad smell.
This type of cellulose assimilatory denitrification with wood chips is much easier to control as there can be a separate filter such as a second canister which does only the oxidation of ammonia to nitrate.
Also note there a million variations which can be made to this design. One can make this a slow flow reactor, where only drops of water per minute are added to the aquarium (typically one uses a dosing pump on an hourly timer). This makes this an anoxic reactor which can reduce nitrates to nitrogen gas. But this type of filter is tricky. It is difficult to avoid smells. and sometimes nitrites will be released.

Removing Nitrates with Sugar
Nitrate can be tied up in brown gunk in a sponge filter media if the food fed has a high amount of carbohydrates in it. One sets up an aquarium with three large powerhead operated sponge filters, three thick layers of 20 ppi foam in a large canister filter or three thick layers of 20 ppi foam in a sump and runs it for two months. One adds a carbohydrate such as sugar at about 10% of the level of the food added daily.
Then after two months of operation ONE of the filters is thoroughly cleaned of the nitrogen filled brown gunk in the sponge. At three months a second sponge is cleaned. And at four months a third sponge is cleaned. The process is then repeated. This means a sponge will operate for three months between cleanings.

This will ONLY work with a very large amount of sponge per gram of fish. If there is not a large amount of sponge the water will become filled with sugar which in turn will give lots of bacteria and a toxic environment for fish. This process is more challenging than the wood chip method as the carbohydrates are put into the water as soluble sugar.
This means any filter on the aquarium will build up brown gunk and clog. So one runs the risk of losing ammonia oxidation in the aquarium. It is far better to just set this up with the sponge/foam but without the sugar. This typically will only reduce nitrate buildup. It will typically not eliminate nitrate build up. But it is a lot easier and a lot safer for the fish.

.
Return to Filter Menu
.
Aquarium Science Website
The chapters shown below or on the right side in maroon lead to close to 400 articles on all aspects of keeping a freshwater aquarium. These articles have NO links to profit making sites and are thus unbiased in their recommendations, unlike all the for-profit sites you will find with Google. Bookmark and browse!
.

Korhaka says
Partly an update from my comment on 8.9, I do now suspect what is going on is most likely assimilatiory denitrification. In 5 days nitrates have roughly halved in a 1L test bottle. As it was in a sealed bottle if this is assimilatory denitrification I would expect that at some point oxygen will deplete or already has done. The source of marsh sludge likely also acted as its own carbon source given that it is made up of mostly decaying leaves, in addition to being filled with bacteria.
If anything the most interesting thing here so far is that the bacteria are able to make a noticeable difference after just 5 days – potentially less as this was my first time opening and testing it. There was also minimal water agitation other than shaking the bottle once a day. If I can I would like to repeat this while adding oxygen. This could potentially be useful as a very quick way of getting some form of garbage can denitrification setup running.
Aware that my water isn’t too bad at 40ppm nitrate, but I find that the garden shed biology experiments are rather fun and I might be able to get something productive out of it.
Dave says
In reply to Katherine … All your nitrate reductions are due to some combination of assimilatory denitrification. Whether it is plants, algae, wood, bacteria, mulm buildup, or some combination is impossible to tell.
Katherine says
So fascinating! Long response, but I’m also a nerd. I have had 3 tanks so far that have acheived the magic of no nitrates. I don’t think it is plants alone, as my other tanks with just as many plants build up nitrates like mad. I’m curious what you think created this magic, as I cannot replicate it on demand.
One tank had probably a 2″ sand capped gravel substrate. Gas did build up in the substrate, and I theorized the magic might be happening there. It also had quite a bit of driftwood, though I wouldn’t say it appeared to be actively “rotting.”
My other tank I used one of caribsea’s straw type media under a planted/active substrate, also with driftwood. Potentially it was that substrate additive?
My third tank was a small 6g shrimp tank, no driftwood, also an active substrate; but algae would build up on the walls pretty severely and need cleaned. I figured the shrimp enjoyed this, but they never seemed to clean it themselves.
I have another 75g tank with some wood and plants that accumulates algae on the glass similarly – though it’s more likely brown algae or the green kind that clings to the sand and falls apart when touched, in comparison to the green that only stuck to the glass in my shrimp tank. It always accumulates significant nitrates.
Please tell me what you think! I was preparing to set up another tank and test the bioballs underneath an undergravel filter/style system and see what happens, but it sounds like it may just be a waste of my time per the science you’ve reported!
Dave says
In reply to Lycan …. You are correct. Rotting driftwood can also create assimilatory denitrification.
Lycan Thea says
Instead of using wood shavings, would driftwood work to induce the same process? I have an old piece of driftwood that is slowly rotting away in the tank, and I have 0 nitrates. Is the driftwood creating the high carbon conditions necessary for this type of denitrification to happen?